Anti-Human IGF1 Receptor Recombinant Antibody (Cixutumumab) (CAT#: TAB-078)
Recombinant monoclonal antibody to IGF1 Receptor. Cixutumumab is a monoclonal antibody for the treatment of solid tumors.
It is a fully human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor (IGF-1R) with potential antineoplastic activity. Cixutumumab selectively binds to membrane-bound IGF-1R, thereby preventing the binding of the natural ligand IGF-1 and the subsequent activation of PI3K/AKT signaling pathway. Downregulation of the PI3K/AKT survival pathway may result in the induction of cancer cell apoptosis and may decrease cancer cellular proliferation. IGF-1R, a receptor tyrosine kinase of the insulin receptor superfamily overexpressed by many cancer cell types, stimulates cell proliferation, enables oncogenic transformation, and suppresses apoptosis; IGF-1R signaling has been implicated in tumorigenesis and metastasis.

Figure 1 Effects of bevacizumab, cixutumumab, and combination bevacizumab and cixutumumab in vitro and in vivo.
A, Western blotting for IGF-1 receptor b, VEGFR-2, and phospho-IGF-1 receptor b in HUVEC cells treated with cixutumumab (20 mg/mL), bevacizumab (75 mg/mL), and the combination of cixutumumab and bevacizumab. GAPDH was used as control. B, Western blotting for IGF-1 receptor b, VEGFR-2, and p-IGF-1 receptor b in SKOV3 cells treated with cixutumumab (20 mg/mL), bevacizumab (75 mg/mL), and the combination. GAPDH was used as control. C, growth curve for SKOV3 xenografts. Results are means AE SE of 6 mice per group; P < 0.05 for overall Kruskal-Wallis test at week 6; cixutumumab was different from the combination therapy. D, growth curve for A2780 xenografts. Results are means AE SE of 5 mice per group, P < 0.05 for overall ANOVA test at day 17. Control was different from all other groups and cixutumumab alone was different from bevacizumab and the combination therapy.
Shao, M., Hollar, S., Chambliss, D., Schmitt, J., Emerson, R., Chelladurai, B.,... & Matei, D. (2012). Targeting the insulin growth factor and the vascular endothelial growth factor pathways in ovarian cancer. Molecular cancer therapeutics, 11(7), 1576-1586.

Figure 2 MVD and apoptosis in SKOV3 xenografts treated with control, bevacizumab, cixutumumab, and combination bevacizumab and cixutumumab.
A, representative immunohistochemical staining for CD31 shows microvessel density in control, cixutumumab, bevacizumab, and combination-treated xenografts (Â100 magnification). B, quantification of microvessel counts was carried out in 5 HPF per specimen in the 4 groups. Results are means of counts in 5 HPF AE SE of 3 specimens per group; results of overall Kruskal-Wallis test were not statistically significant; the 2 bevacizumab groups combined were different from control. C, representative TUNEL immunofluorescent staining in control-, cixutumumab-, bevacizumab-, and combinationtreated xenografts. D, quantification of positive cells in the TUNEL assay.
Shao, M., Hollar, S., Chambliss, D., Schmitt, J., Emerson, R., Chelladurai, B.,... & Matei, D. (2012). Targeting the insulin growth factor and the vascular endothelial growth factor pathways in ovarian cancer. Molecular cancer therapeutics, 11(7), 1576-1586.

Figure 3 Immunohistochemistry for Ki67 and phospho-Ser 473-AKT in control-, cixutumumab-, bevacizumab-, and combinationtreated SKOV3 xenografts.
A, representative immunohistochemical staining for Ki67 in control-, cixutumumab-, bevacizumab-, and combinationtreated xenografts (Â100 magnification). B, quantification of Ki67-positive cells was carried out in 5 HPF per specimen in the 4 groups (n ¼ 3 specimens per group). The overall ANOVA test was significant and all treatment groups were different from control. Representative C, immunohistochemical staining for phosphoSer 473-AKT in control-, cixutumumab-, bevacizumab-, and combination-treated xenografts (Â100 magnification). D, H scores were calculated as described in Materials and Methods. Results are means AE SE of 3 specimens per group; the overall ANOVA test was not statistically significant.
Shao, M., Hollar, S., Chambliss, D., Schmitt, J., Emerson, R., Chelladurai, B.,... & Matei, D. (2012). Targeting the insulin growth factor and the vascular endothelial growth factor pathways in ovarian cancer. Molecular cancer therapeutics, 11(7), 1576-1586.

Figure 4 HNSCC and NSCLC cell lines display differential sensitivities to cixutumumab in the 3D mimic condition.
Indicated HNSCC and NSCLC cells cultured in poly(HEMA)-coated plates (PCP) and in ultralow attached plates (UAP) were treated with hIgG1 (25 μg/mL) or IMC-cixutumumab (25 μg/mL) for 3 (A, C, and D) or 5 days (B) in the presence of FBS or for 6 hours in the absence of FBS and then stimulated with 10% FBS for 30 minutes (A, bottom). A, representative morphologies of LN686 and OSC19 cells (Con, control; Cixu, cixutumumab). A (bottom) and D, Western blot was done for the indicated proteins.
Shin, D. H., Min, H. Y., El-Naggar, A. K., Lippman, S. M., Glisson, B., & Lee, H. Y. (2011). Akt/mTOR Counteract the Antitumor Activities of Cixutumumab, an Anti-Insulin–like Growth Factor I Receptor Monoclonal Antibody. Molecular cancer therapeutics, 10(12), 2437-2448.

Figure 5 Cixutumumab induced the activities and expression levels of EGFR and Akt is through mTOR-mediated protein synthesis.
A, RT-PCR analysis of LN686 and FADU cells grown in PCPs in the presence of vehicle (Con) or cixutumumab (Cixu) for 3 days. B, LN686 cells grown in PCP were treated with cixutumumab in the absence of FBS for indicated time period and then stimulated with 10% FBS for 30 minutes before harvest. The indicated proteins were detected by Western blot analysis. C, LN686 cells grown in PCP in the presence of vehicle (Con) or cixutumumab (Cixu) with or without rapamycin (Rapa) or 3 days were metabolically pulse-labeled with trans 35S-methionine and cysteine and then chased with media containing methionine and cysteine for the indicated time periods.
Shin, D. H., Min, H. Y., El-Naggar, A. K., Lippman, S. M., Glisson, B., & Lee, H. Y. (2011). Akt/mTOR Counteract the Antitumor Activities of Cixutumumab, an Anti-Insulin–like Growth Factor I Receptor Monoclonal Antibody. Molecular cancer therapeutics, 10(12), 2437-2448.

Figure 6 Interaction between cixutumumab-treated cancer cells and stromal cells stimulates tumour angiogenesis.
(a) Representative images of migration and tube formation assay of cixutumumab-treated HUVECs. (b) HUVECs were seeded in the top chamber of the transwell insert and allowed to migrate for 6 h. Left: cixutumumab-treated H1299 cells were seeded in the bottom chambers of the transwell. Right: CM from cixutumumab-treated H1299 cells were filled in the bottom chambers of the transwell. (c) Co-culture of cancer cells and stromal cells.
Lee, J. S., Kang, J. H., Boo, H. J., Hwang, S. J., Hong, S., Lee, S. C.,... & Kim, B. J. (2015). STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nature communications, 6, 8499.

Figure 7 Transcriptionally upregulated IGF-2 mediates the interaction between cancer cells and stromal cells during IGF-1R blockade.
(a) Indicated cancer cells and stromal cells were treated with cixutumumab for 6 days, and expression of ligands and receptors of the IGF axis were determined by real-time PCR. (b) CM from cixutumumab-treated cancer cells were analysed by western blotting to confirm IGF-2 secretion during cixutumumab treatment. Lower blots present Coomassie Blue (CB) staining as a loading control. Graph below shows densitometric analysis of three independent western blot assays. (c) IGF-2 immunofluorescence staining in cixutumumab-treated MDA231 cells (scale bar, 10 μm). (d) IGF-1R expression was stably reduced by shRNA in H1299 cells, and the expression of IGF-2 and IGF-1R was determined by real-time PCR (left) and western blotting (right). (e) Ligand, receptor and IGF-2R expression in stromal cells were determined by RT–PCR and western blotting. (f, left) RT–PCR and western blotting data revealing altered cixutumumab-induced IGF-2 expression. (f, right) Wi38 or THP-1 cells were seeded in the top chamber of the transwell insert. The bottom chambers were filled with CM from cixutumumab-treated H1299 cells transfected with empty vector or shIGF-2.
Lee, J. S., Kang, J. H., Boo, H. J., Hwang, S. J., Hong, S., Lee, S. C.,... & Kim, B. J. (2015). STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nature communications, 6, 8499.

Figure 8 IGF-1R blockade-induced IGF2 transcription via STAT3 activation.
(a) Protein lysates from cixutumumab-treated H1299 cells were incubated with phospho-RTK arrays. Spots are in duplicate, with each pair corresponding to a specific pRTK. The pair spots in the corners are positive controls. STAT3 phosphorylation was determined by western blotting in (b) cixutumumab-treated or (c) IGF-1R-silenced H1299 cells. (d) Luciferase assay for IGF-2 promoter activity. Insert: STAT3 expression was reduced by shRNA in H1299 cells as confirmed by western blotting. Left: P3 promoter. Right: P4 promoter. Each bar represents the mean relative luciferase unit (RLU) ±s.d. of four identical wells of a single representative experiment. (e) The effect of cixutumumab on IGF-2 expression in H1299 cells with reduced STAT3 expression was determined by RT–PCR and western blotting. (f) Wi38 cells were seeded in the top chamber of the transwell insert. Cixutumumab-treated H1299 cells transfected with empty vector or shSTAT3 expression were seeded in the bottom chambers of the transwell. Wi38 cells were allowed to migrate for 16 h. Each bar represents the mean relative unit (RU) ±s.d. of three replicates of a single representative experiment.(i) The excised primary tumours were assessed by immunofluorescence staining using anti-F4/80 (left) and -CD34 (right) antibodies (Scale bar, 50 μm). Data are presented as mean positive cells per field of view (FOV) ±s.d.
Lee, J. S., Kang, J. H., Boo, H. J., Hwang, S. J., Hong, S., Lee, S. C.,... & Kim, B. J. (2015). STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nature communications, 6, 8499.

Figure 9 Increased CXCL8 production from stromal cells through the IGF-2/IGF-2R interaction.
(a) Wi38 or THP-1 cells were co-cultured with cixutumumab-treated cancer cells in the transwell for 24 h and the mRNA level of each target was determined by real-time PCR. (b) Wi38 or THP-1 cells were treated with rhIGF-2 (100 ng ml−1, 24 h) and CXCL8 mRNA levels were examined by real-time PCR. (c) IGF-2R expression was reduced by shRNA (left) or anti-IGF-2R-neutralizing antibody (10 μg ml−1) (right) in Wi38 cells and followed by treatment with CM from cixutumumab-treated cancer cells for 24 h. CXCL8 mRNA levels were determined by real-time PCR. (d) Wi38 cells were incubated with CM from cixutumumab-treated H1299 transfected with empty vector or shIGF-2 (left) or anti-IGF-2-neutralizing antibody (5 μg ml−1) and CM from cixutumumab-treated H1299 cells (right). After 24 h, CXCL8 mRNA levels were examined by real-time PCR. (e, left) Wi38 cells were transfected with negative control siRNA or siCXCL8 and knockdown of CXCL8 was confirmed by real-time PCR. (e, right) Transfected with each siRNA were seeded in the bottom chamber and co-cultured with cixutumumab-treated H1299 cells in the transwell.
Lee, J. S., Kang, J. H., Boo, H. J., Hwang, S. J., Hong, S., Lee, S. C.,... & Kim, B. J. (2015). STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nature communications, 6, 8499.

Figure 10 Clinical relevance of tumour-associated macrophages and fibroblasts in HNSCC patients with cixutumumab treatment.
(a) Representative images of IHC analyses on IGF2 expression, VEGFR+ VE cells, F4/80+ macrophages and FSP-1+ fibroblasts in the tissue samples from patient with HNSCC, either naive or treated with cixutumumab for 3 weeks. Images are from patient 1 of each group. Other images are included in Supplementary 9. (b) Schematic model of events noted in the TME on treatment with IGF-1R-targeted therapy.
Lee, J. S., Kang, J. H., Boo, H. J., Hwang, S. J., Hong, S., Lee, S. C.,... & Kim, B. J. (2015). STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nature communications, 6, 8499.
Specifications
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Human
- Derivation
- Human
- Type
- IgG1 - lambda
- Specificity
- Tested positive against native human antigen.
- Species Reactivity
- Human
- Applications
- IP, Neut, FuncS, ELISA, FC, WB, IHC, Stim, IF, Block
- CAS
- 947687-12-9
- Generic Name
- Cixutumumab
- UNII
- 2285XW22DR
- MW
- 146.3 kDa
- Related Disease
- Non-small cell lung cancers (NSCLC)
Product Property
- Purity
- >95.0%, determined by analysis by RP-HPLC & analysis by SDS-PAGE.
- Storage
- Store at 4°C for up to 3 months. For longer term storage aliquot into small volumes and store at -20°C.
Applications
- Application Notes
- The IGF1R antibody has been reported in applications of IP, Neut, FuncS, ELISA, FC, WB, IHC, Stim, IF, Block.
Target
- Alternative Names
- Cixutumumab;947687-12-9;IMC-A12;NSC742460;IGF1R;insulin-like growth factor 1 receptor;CD221;IGFIR;IGFR;JTK13;MGC18216;IGF-I receptor;soluble IGF1R variant 1;soluble IGF1R variant 2;insulin-like growth factor I receptor;MGC142170;MGC142172;
- Gene ID
- 3480
- UniProt ID
- P08069
Related Resources
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Downloads
Download resources about recombinant antibody development and antibody engineering to boost your research.
See other products for "Cixutumumab"
Afuco™ Anti-IGF1R ADCC Recombinant Antibody (Cixutumumab), ADCC EnhancedThis product is an ADCC enhanced antibody produced by our Afuco™ platform. Recombinant monoclonal antibody to IGF1 Receptor. Cixutumumab is a monoclonal antibody for the treatment of solid tumors.
It is a fully human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor (IGF-1R) with potential antineoplastic activity. Cixutumumab selectively binds to membrane-bound IGF-1R, thereby preventing the binding of the natural ligand IGF-1 and the subsequent activation of PI3K/AKT signaling pathway. Downregulation of the PI3K/AKT survival pathway may result in the induction of cancer cell apoptosis and may decrease cancer cellular proliferation. IGF-1R, a receptor tyrosine kinase of the insulin receptor superfamily overexpressed by many cancer cell types, stimulates cell proliferation, enables oncogenic transformation, and suppresses apoptosis; IGF-1R signaling has been implicated in tumorigenesis and metastasis.
See other products for "IGF1R"
Single-domain Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NABL-091 | Recombinant Anti-human IGF1R VHH Single Domain Antibody | WB, ELISA, IHC, FC, FuncS | Llama VHH |
| TAB-096ZJ | Anti-Human IGF-1R Therapeutic Single Domain Antibody (IGF1 R-3) | ELISA, IHC, IF, WB | Single domain antibody |
| HPAB-1545-FY | Recombinant Llama Anti-IGF1R Single Domain Antibody (m632) | WB, ELISA | Llama VHH |
| HPAB-1546-FY | Recombinant Llama Anti-IGF1R Single Domain Antibody (m636) | WB, ELISA | Llama VHH |
| HPAB-1547-FY | Recombinant Llama Anti-IGF1R Single Domain Antibody (m546) | WB, ELISA | Llama VHH |
Human Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-199 | Human Anti-IGF1R Recombinant Antibody (TAB-199) | FC, IP, ELISA, Neut, FuncS, IF, ICC | Human IgG1, κ |
| TAB-209 | Anti-Human IGF1 Receptor Recombinant Antibody (Robatumumab) | FuncS, IF, Neut, ELISA, FC, IP, ICC | IgG1 - kappa |
| TAB-055ZJ-F(E) | Human Anti-IGF1R Recombinant Antibody; Fab Fragment (TAB-055ZJ-F(E)) | ELISA | Human Fab |
| TAB-056ZJ-F(E) | Human Anti-IGF1R Recombinant Antibody; Fab Fragment (TAB-056ZJ-F(E)) | ELISA | Human Fab |
| TAB-057ZJ-F(E) | Human Anti-IGF1R Recombinant Antibody; Fab Fragment (TAB-057ZJ-F(E)) | ELISA | Human Fab |
Humanized Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-736 | Anti-IGF1R Recombinant Antibody (Dalotuzumab) | FC, IP, ELISA, Neut, FuncS, IF, ICC | IgG1 - kappa |
| TAB-035ZJ | Human Anti-IGF1R Recombinant Antibody (TAB-035ZJ) | ELISA, FC | Humanized IgG |
| TAB-036ZJ | Anti-Human IGF-1R Recombinant Antibody (H0L0 IgGIm(AA)) | ELISA, Neut, FC, IHC | Humanized antibody |
| TAB-037ZJ | Anti-Human IGF-1R Recombinant Antibody (H1L0 IgGIm(AA)) | ELISA, Neut, FC, IHC | Humanized antibody |
| TAB-038ZJ | Human Anti-IGF1R Recombinant Antibody (TAB-038ZJ) | FuncS, IP, Inhib, IF | Humanized IgG1 |
Immunotoxin
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-L024E | IGF1-PE immunotoxin | Cytotoxicity assay, Functional assay | |
| AGTO-L024D | IGF1-DT immunotoxin | Cytotoxicity assay, Functional assay |
Mouse Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-009ZJ | Mouse Anti-IGF1R Recombinant Antibody (TAB-009ZJ) | ELISA, FC | Mouse IgG1, κ |
| TAB-010ZJ | Anti-Human IGF-1R Recombinant Antibody (6E11) | ELISA, Neut, FC, IHC | |
| TAB-011ZJ | Mouse Anti-IGF1R Recombinant Antibody (TAB-011ZJ) | ELISA, FC, WB, Inhib | Mouse IgG2, κ |
| TAB-012ZJ | Mouse Anti-IGF1R Recombinant Antibody (TAB-012ZJ) | ELISA, FC, WB, Inhib | Mouse IgG2, κ |
| TAB-016ZJ-F(E) | Anti-Human IGF-1R Recombinant Antibody Fab Fragment (7C2) | IP, IHC, ELISA, FC |
Chimeric Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-084ZJ-S(P) | Mouse Anti-IGF1R Recombinant Antibody; scFv Fragment (TAB-084ZJ-S(P)) | ELISA, FC | Mouse scFv |
| TAB-085ZJ-S(P) | Anti-Human IGF-1R Recombinant Antibody scFv Fragment (6E11c) | ELISA, Neut, FC, IHC | Chimeric antibody (mouse/human) |
| TAB-086ZJ-S(P) | Mouse Anti-IGF1R Recombinant Antibody; scFv Fragment (TAB-086ZJ-S(P)) | ELISA, FC, Inhib, IP | Mouse scFv |
| TAB-087ZJ-S(P) | Anti-Human IGF-1R Recombinant Antibody scFv Fragment (ch7C2) | ELISA | Chimeric antibody (mouse/human) |
| TAB-093ZJ-S(P) | Anti-Human IGF-1R Recombinant Antibody scFv Fragment (ch9E11) | ELISA | Chimeric antibody (mouse/human) |
Chicken IgY Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| BRD-0103MZ | Chicken Anti-CD221 Polyclonal IgY | WB | Chicken antibody |
Neutralizing Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1075CQ | Mouse Anti-IGF1R Recombinant Antibody (clone alphaIR3) | FC, IP, Neut, ICC, IF | Mouse IgG1 |
| NEUT-1076CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 33255.111) | ELISA, Neut, WB | Mouse IgG1 |
| NEUT-1081CQ | Mouse Anti-IGF1R Recombinant Antibody (clone CBL453) | WB, ELISA, Neut | Mouse IgG1 |
| NEUT-1082CQ | Mouse Anti-IGF1R Recombinant Antibody (clone CBL080) | Neut, WB | Mouse IgG1 |
Blocking Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1077CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 1H7) | FC, Block, IHC, IP, WB | Mouse IgG1, κ |
| NEUT-1078CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 24-60) | Inhib, ICC, IF, IP, WB | Mouse IgG2a, κ |
| NEUT-1079CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 17-69) | Inhib, IP | Mouse IgG1 |
| NEUT-1080CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 24-57) | Inhib, IP | Mouse IgG1, κ |
Rabbit Monoclonal Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-1755 | Rabbit Anti-IGF1R Recombinant Antibody (clone DS1755AB) | IP, ELISA | Rabbit IgG |
| MOR-4684 | Rabbit Anti-IGF1R Recombinant Antibody (clone TH198DS) | WB, ELISA | Rabbit IgG |
| MOR-4685 | Rabbit Anti-IGF1R Recombinant Antibody (clone TH199DS) | WB | Rabbit IgG |
Neuroscience Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NS-041CN | Mouse Anti-IGF1R Recombinant Antibody (NS-041CN) | ELISA, WB, FC | Mouse IgG |
| NS-041CN-F(E) | Mouse Anti-IGF1R Recombinant Antibody; Fab Fragment (NS-041CN-F(E)) | ELISA, WB, FC | Mouse Fab |
| NS-041CN-S(P) | Mouse Anti-IGF1R Recombinant Antibody; scFv Fragment (NS-041CN-S(P)) | ELISA, WB, FC | Mouse scFv |
Recombinant Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0210CQ | Human Anti-IGF1R Recombinant Antibody (clone 15H12) | ELISA, FC, Neut | Human IgG3, κ |
| HPAB-0211CQ | Human Anti-IGF1R Recombinant Antibody (clone 1H3) | ELISA, FC, Neut | Human IgG |
| HPAB-0212CQ | Human Anti-IGF1R Recombinant Antibody (clone 11A4) | ELISA, FC, Block | Human IgG1 |
| HPAB-0213CQ | Human Anti-IGF1R Recombinant Antibody (clone 8A1) | ELISA, FC, Block | Human IgG1 |
| HPAB-0214CQ | Human Anti-IGF1R Recombinant Antibody (clone PINT-9A2) | ELISA, FC, Block | Human IgG1 |
Fab Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0210CQ-F(E) | Human Anti-IGF1R Recombinant Antibody (clone 15H12); Fab Fragment | ELISA, FC, Neut | Human Fab |
| HPAB-0211CQ-F(E) | Human Anti-IGF1R Recombinant Antibody (clone 1H3); Fab Fragment | ELISA, FC, Neut | Human Fab |
| HPAB-0212CQ-F(E) | Human Anti-IGF1R Recombinant Antibody (clone 11A4); Fab Fragment | ELISA, FC, Block | Human Fab |
| HPAB-0213CQ-F(E) | Human Anti-IGF1R Recombinant Antibody (clone 8A1); Fab Fragment | ELISA, FC, Block | Human Fab |
| HPAB-0214CQ-F(E) | Human Anti-IGF1R Recombinant Antibody (clone PINT-9A2); Fab Fragment | ELISA, FC, Block | Human Fab |
ADCC Enhanced Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-199 | Afuco™ Anti-IGF1R Recombinant Antibody (AFC-TAB-199), ADCC Enhanced | FC, IP, ELISA, Neut, FuncS, IF | Human IgG1, κ |
| AFC-TAB-209 | Afuco™ Anti-IGF1R ADCC Recombinant Antibody (Robatumumab), ADCC Enhanced | FuncS, IF, Neut, ELISA, FC, IP | ADCC enhanced antibody |
| AFC-TAB-736 | Afuco™ Anti-IGF1R ADCC Recombinant Antibody (Dalotuzumab), ADCC Enhanced | FC, IP, ELISA, Neut, FuncS, IF | ADCC enhanced antibody |
| AFC-TAB-078 | Afuco™ Anti-IGF1R ADCC Recombinant Antibody (Cixutumumab), ADCC Enhanced | IF, IP, Neut, FuncS, ELISA, FC | ADCC enhanced antibody |
| AFC-TAB-232 | Afuco™ Anti-IGF1R ADCC Recombinant Antibody (Figitumumab), ADCC Enhanced | IF, IP, Neut, FuncS, ELISA | ADCC enhanced antibody |
scFv Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0839-FY-F(E) | Mouse Anti-IGF1R Recombinant Antibody; Fab Fragment (HPAB-0839-FY-F(E)) | IHC | Mouse Fab |
| HPAB-0840-FY-F(E) | Mouse Anti-IGF1R Recombinant Antibody; Fab Fragment (HPAB-0840-FY-F(E)) | IHC | Mouse Fab |
| HPAB-0841-FY-F(E) | Mouse Anti-IGF1R Recombinant Antibody; Fab Fragment (HPAB-0841-FY-F(E)) | IHC | Mouse Fab |
| HPAB-1548-FY-F(E) | Human Anti-IGF1R Recombinant Antibody; Fab Fragment (HPAB-1548-FY-F(E)) | ELISA | Human Fab |
| HPAB-2063-FY-S(P) | Human Anti-IGF1R Recombinant Antibody; scFv Fragment (HPAB-2063-FY-S(P)) | IA | Human scFv |
Customer Reviews and Q&As
There are currently no Customer reviews or questions for TAB-078. Click the button above to contact us or submit your feedback about this product.
View the frequently asked questions answered by Creative Biolabs Support.
For Research Use Only. Not For Clinical Use.
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.









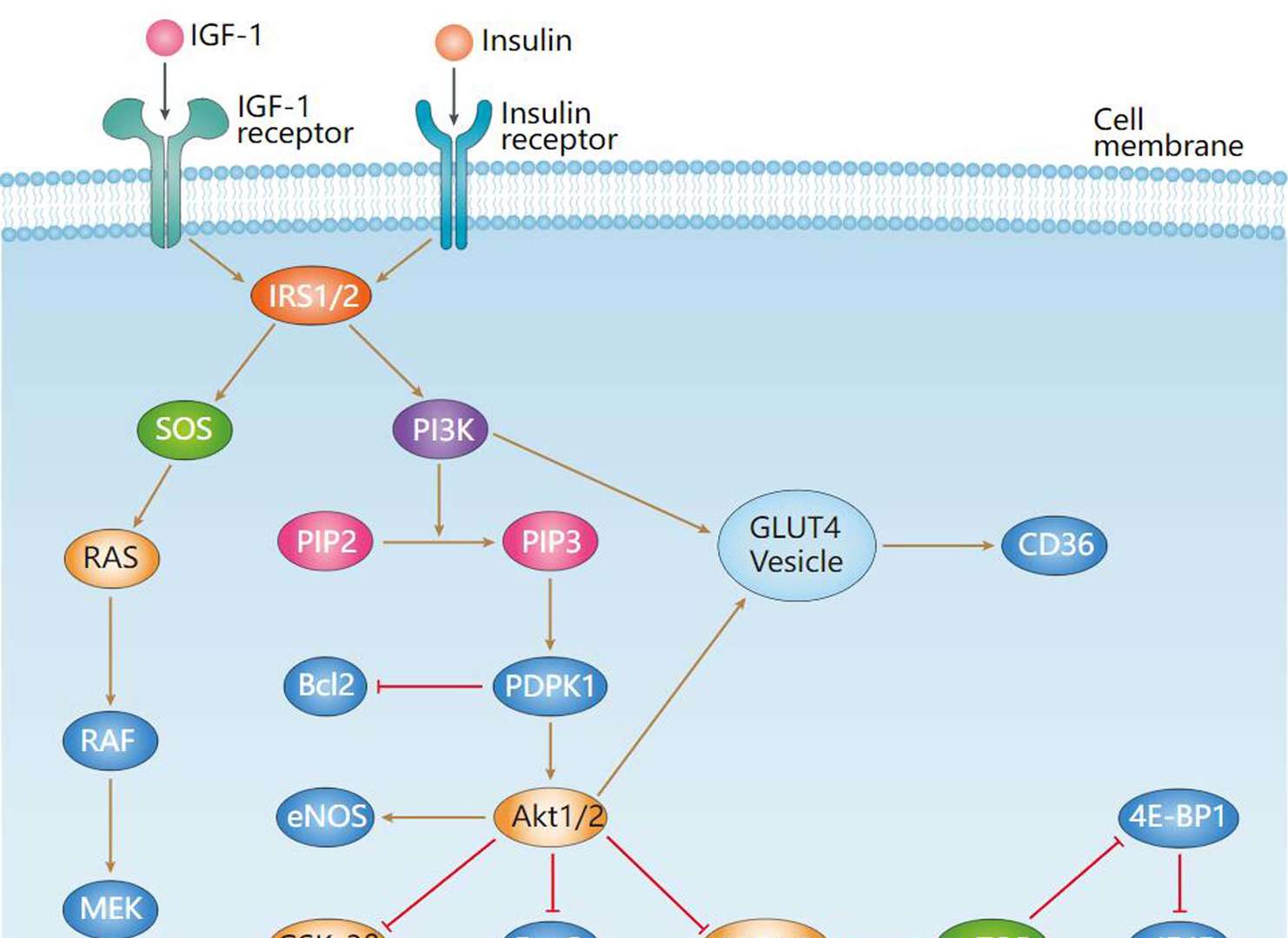 Insulin Signaling Pathway
Insulin Signaling Pathway
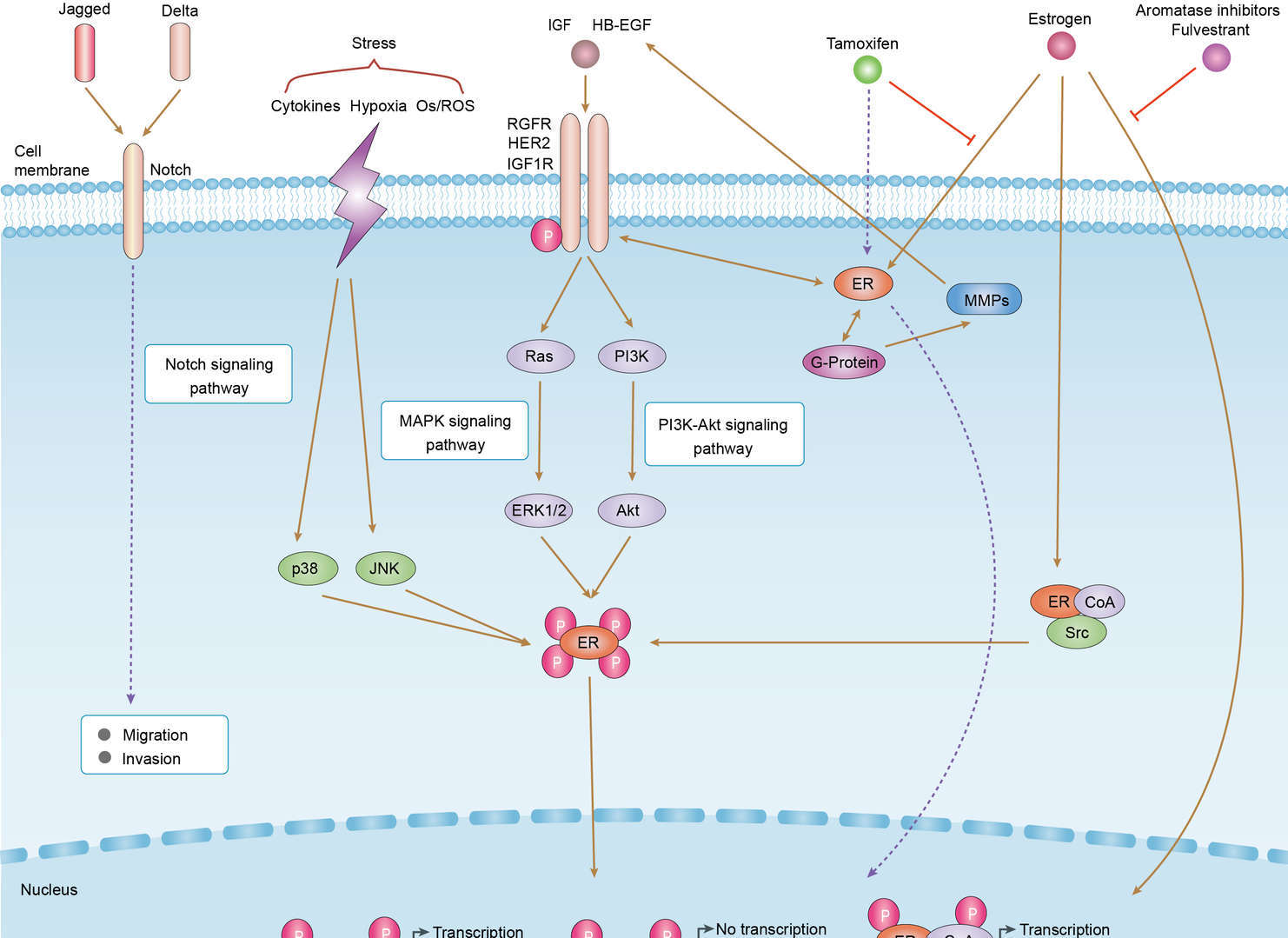 Endocrine Resistance
Endocrine Resistance
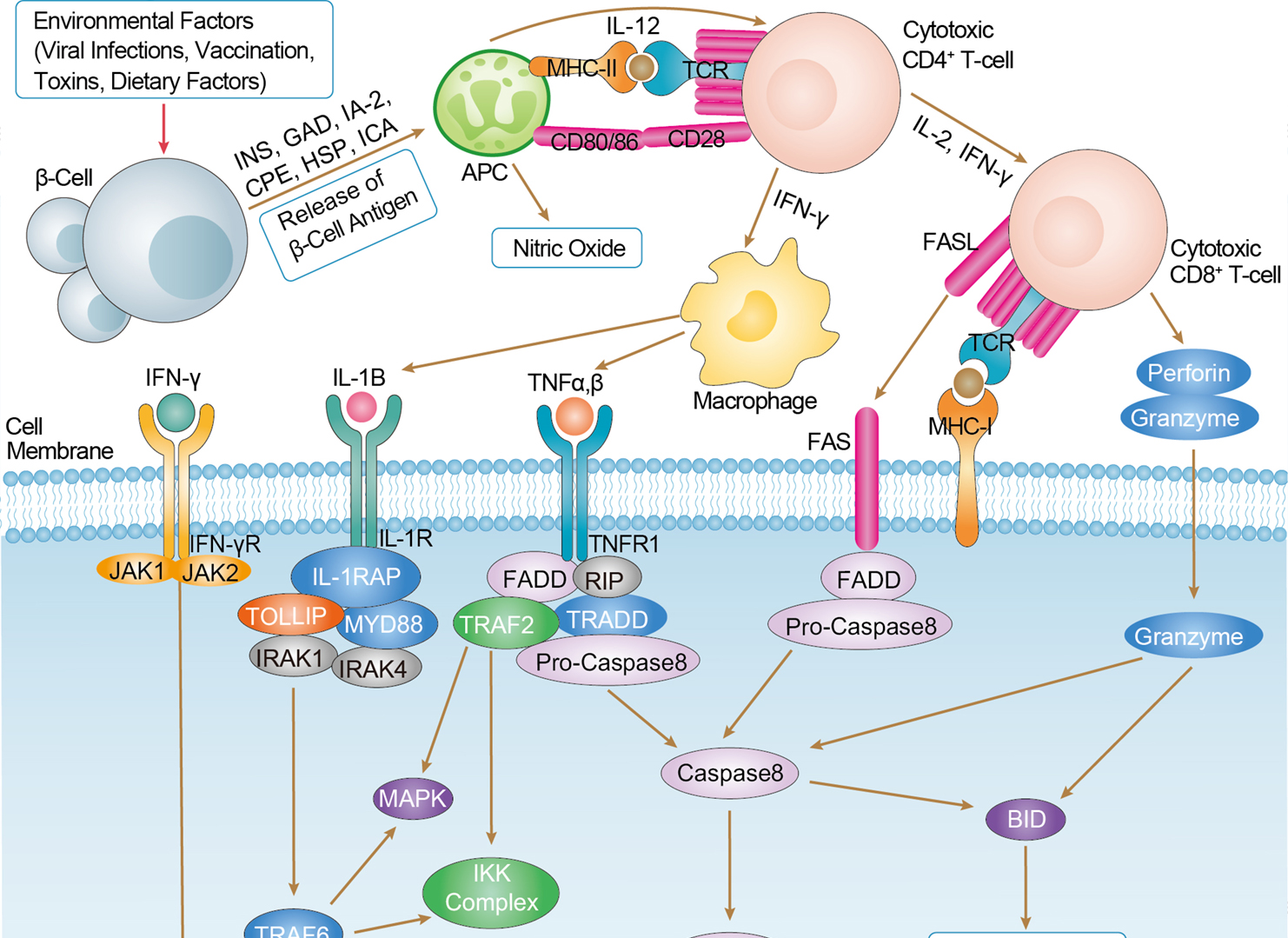 Type I Diabetes Mellitus
Type I Diabetes Mellitus
