Afuco™ Anti-Human IL13 ADCC Recombinant Antibody (RPC4046), ADCC Enhanced (CAT#: AFC-379CL)
Anti-IL13 ADCC Enhanced Antibody (RPC4046) is an ADCC enhanced antibody produced by our Afuco™ platform. RPC4046 is a monoclonal antibody currently being studied in a Phase 2 trial for the treatment of eosinophilic esophagitis (EoE). RPC4046 is directed against the interleukin-13 (IL-13) target, which has been validated in other allergy-related disorders including asthma. The development program for RPC4046 builds upon competencies in immunology and GI diseases.

Figure 1 At week 16, mean reductions from baseline in esophageal eosinophil count were 4.42 ± 59.94, 94.76 ± 67.27, and 99.90 ± 79.53 eos/hpf in the placebo, 180 mg RPC4046, and 360 mg RPC4046 groups, respectively.
Mean changes between either RPC4046 dose and placebo were statistically significant at week 16 (P < .0001 for both comparisons; mean counts). Peak esophageal eosinophil counts were significantly reduced, with 50% of patients treated with 180 mg and 360 mg having <15 peak eos/hpf compared with 0% placebo (P < .0001 for both comparisons), and 25% of patients in the 180 mg RPC4046 group and 20% in the 360 mg RPC4046 group having <6 peak eos/hpf after treatment (P = .0027 and P = .0079, respectively).
Hirano, I., Collins, M. H., Assouline-Dayan, Y., Evans, L., Gupta, S., Schoepfer, A. M., ... & Tompkins, C. A. (2019). RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology, 156(3), 592-603.

Figure 2 For overall EoE patient group, mean patient’s global assessment of disease severity was significantly reduced at week 16 for the 360-mg RPC4046 group compared with placebo.
Mean reductions in the patient's global assessment of disease severity score of 2.01 ± 1.68 and 2.8 ± 2.71 were significant at week 16 with the 360-mg dose (n = 27, P = .0107) but not with the 180-mg dose (n = 30, P = .1035) (Figure A). Mean reductions in the clinician's global assessment of disease severity score of 3.6 ± 2.71 and 2.9 ± 2.70 were significant at week 16 with the 180-mg (n = 27, P = .0094) and 360-mg doses (n = 30, P = .0352) (Figure B). Categorical analysis of patient's global impression of change in EoE symptoms at week 16 did not show significant improvements in either RPC4046 group (180 mg: n = 31, P = .4094; 360 mg: n = 34, P = .0143) (Figure C).
Hirano, I., Collins, M. H., Assouline-Dayan, Y., Evans, L., Gupta, S., Schoepfer, A. M., ... & Tompkins, C. A. (2019). RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology, 156(3), 592-603.
Specifications
- Host Species
- Humanized
- Derivation
- Humanized
- Type
- ADCC enhanced antibody
- Species Reactivity
- Human
- Related Disease
- Eosinophilic Esophagitis
Product Property
- Purity
- >97%, by SDS-PAGE under reducing conditions
- Storage
- Store at -20°C for long-term storage. Store at 4°C for up to one month. Avoid freeze/thaw cycles.
Target
Related Resources
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Downloads
Download resources about recombinant antibody development and antibody engineering to boost your research.
See other products for "IL13"
Recombinant Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1287z | Mouse Anti-IL13 Recombinant Antibody (clone 39B4) | WB, IP, IF, ELISA | Mouse IgG1, κ |
| PABW-170 | Human Anti-IL13 Recombinant Antibody (clone CNTO607) | ELISA, WB, Neut | Human IgG |
| MOB-0781CT | Recombinant Mouse anti-Human IL13 Monoclonal antibody (43227.22) | ELISA, Neut, WB | |
| HPAB-0045LY | Human Anti-IL13 Recombinant Antibody (clone IMA-026) | WB, ELISA, FC, IF | Humanized IgG1 |
| HPAB-AP106-YC | Mouse Anti-IL13 Recombinant Antibody (HPAB-AP106-YC) | WB, ELISA, FC, IHC | Mouse IgG |
Human Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-218 | Anti-Human IL13 Recombinant Antibody (Tralokinumab) | ELISA, FC, IP, FuncS, IF, Neut, ICC | IgG4 - lambda |
| TAB-067ZJ-F(E) | Anti-Human IL-13 Recombinant Antibody Fab Fragment (CNTO 607) | ELISA, Inhib | Human antibody |
| TAB-068ZJ-F(E) | Anti-Human IL-13 Recombinant Antibody Fab Fragment (01471/G6) | ELISA, Neut | Human antibody |
| TAB-069ZJ-F(E) | Human Anti-IL13 Recombinant Antibody; Fab Fragment (TAB-069ZJ-F(E)) | ELISA, Neut, FC | Human Fab |
| TAB-070ZJ-F(E) | Human Anti-IL13 Recombinant Antibody; Fab Fragment (TAB-070ZJ-F(E)) | ELISA, Neut, FC | Human Fab |
Humanized Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-H05 | Anti-Human IL13 Recombinant Antibody (Anrukinzumab) | ELISA, Inhib | IgG1 |
| TAB-043ZJ | Anti-Human IL-13 Recombinant Antibody (Lc6Hc2) | ELISA | Humanized antibody |
| TAB-044ZJ | Human Anti-IL13 Recombinant Antibody (TAB-044ZJ) | ELISA, Neut | Humanized IgG |
| TAB-045ZJ | Human Anti-IL13 Recombinant Antibody (TAB-045ZJ) | ELISA, Block | Humanized IgG |
| TAB-046ZJ | Human Anti-IL13 Recombinant Antibody (TAB-046ZJ) | ELISA, Block | Humanized IgG |
scFv Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-600 | Human Anti-IL13 Recombinant Antibody (clone H2L6); scFv Fragment | ELISA | Humanized scFv |
| PSBL-601 | Mouse Anti-IL13 Recombinant Antibody (clone X836); scFv Fragment | WB, ELISA, FuncS | Mouse scFv |
| HPAB-0286-YJ-S(P) | Human Anti-IL13 Recombinant Antibody; scFv Fragment (HPAB-0286-YJ-S(P)) | ELISA, Antagonist, FuncS | Human scFv |
| HPAB-0287-YJ-S(P) | Mouse Anti-IL13 Recombinant Antibody; scFv Fragment (HPAB-0287-YJ-S(P)) | ELISA, Neut, Inhib, FuncS | Mouse scFv |
| HPAB-1746-FY-F(E) | Human Anti-IL13 Recombinant Antibody; Fab Fragment (HPAB-1746-FY-F(E)) | WB, ELISA | Human Fab |
Mouse Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-022ZJ | Anti-Human IL-13 Recombinant Antibody (C836) | ELISA | |
| TAB-023ZJ | Mouse Anti-IL13 Recombinant Antibody (TAB-023ZJ) | ELISA, Block | Mouse IgG |
| TAB-022ZJ-S(P) | Anti-Human IL-13 Recombinant Antibody scFv Fragment (C836) | ELISA | |
| TAB-022ZJ-F(E) | Anti-Human IL-13 Recombinant Antibody Fab Fragment (C836) | ELISA | |
| TAB-023ZJ-F(E) | Mouse Anti-IL13 Recombinant Antibody; Fab Fragment (TAB-023ZJ-F(E)) | ELISA, Block | Mouse Fab |
Chimeric Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-094ZJ | Anti-Human IL-13 Recombinant Antibody (167) | ELISA | Chimeric antibody (mouse/human) |
| TAB-094ZJ-S(P) | Anti-Human IL-13 Recombinant Antibody scFv Fragment (167) | ELISA | Chimeric antibody (mouse/human) |
| TAB-094ZJ-F(E) | Anti-Human IL-13 Recombinant Antibody Fab Fragment (167) | ELISA | Chimeric antibody (mouse/human) |
Neutralizing Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1171CQ | Mouse Anti-IL13 Recombinant Antibody (clone B-B13) | ELISA, ELISPOT, Neut | Mouse IgG1 |
| NEUT-1172CQ | Mouse Anti-IL13 Recombinant Antibody (clone 10X28) | Neut, WB | Mouse IgG1 |
| NEUT-1173CQ | Mouse Anti-IL13 Recombinant Antibody (clone 15H10) | Neut | Mouse IgG2b |
| NEUT-1174CQ | Mouse Anti-IL13 Recombinant Antibody (clone 32116.11) | ELISA, Neut, WB | Mouse IgG1 |
| NEUT-1175CQ | Rat Anti-IL13 Recombinant Antibody (clone 5A2) | Neut | Rat IgG1 |
Rabbit Monoclonal Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-1790 | Rabbit Anti-IL13 Recombinant Antibody (clone DS1790AB) | WB, ICC, FC, IP | Rabbit IgG |
| MOR-4610 | Rabbit Anti-IL13 Recombinant Antibody (clone TH123DS) | WB | Rabbit IgG |
Fab Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0045LY-F(E) | Human Anti-IL13 Recombinant Antibody (clone IMA-026); Fab Fragment | WB | Humanized Fab |
| HPAB-AP106-YC-F(E) | Mouse Anti-IL13 Recombinant Antibody; Fab Fragment (HPAB-AP106-YC-F(E)) | ELISA | Mouse Fab |
| HPAB-AP107-YC-F(E) | Mouse Anti-IL13 Recombinant Antibody; Fab Fragment (HPAB-AP107-YC-F(E)) | ELISA | Mouse Fab |
| HPAB-AP108-YC-F(E) | Mouse Anti-IL13 Recombinant Antibody; Fab Fragment (HPAB-AP108-YC-F(E)) | ELISA | Mouse Fab |
| HPAB-0286-YJ-F(E) | Human Anti-IL13 Recombinant Antibody; Fab Fragment (HPAB-0286-YJ-F(E)) | ELISA, Antagonist, FuncS | Human Fab |
ADCC Enhanced Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-H05 | Afuco™ Anti-IL13 ADCC Recombinant Antibody (Anrukinzumab), ADCC Enhanced | FC, IP, ELISA, Neut, FuncS, IF | ADCC enhanced antibody |
| AFC-TAB-017ML | Afuco™ Anti-IL13 ADCC Recombinant Antibody (Dectrekumab), ADCC Enhanced | ELISA, IHC, FC, IP, IF, Inhib | ADCC enhanced antibody |
| AFC-TAB-219 | Afuco™ Anti-IL13 ADCC Recombinant Antibody (Lebrikizumab), ADCC Enhanced | ELISA, Neut, IF, IP, FC, FuncS | ADCC enhanced antibody |
| AFC-TAB-218 | Afuco™ Anti-IL13 ADCC Recombinant Antibody (Tralokinumab), ADCC Enhanced | ELISA, FC, IP, FuncS, IF, Neut | ADCC enhanced antibody |
Customer Reviews and Q&As
There are currently no Customer reviews or questions for AFC-379CL. Click the button above to contact us or submit your feedback about this product.
View the frequently asked questions answered by Creative Biolabs Support.
For Research Use Only. Not For Clinical Use.
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.





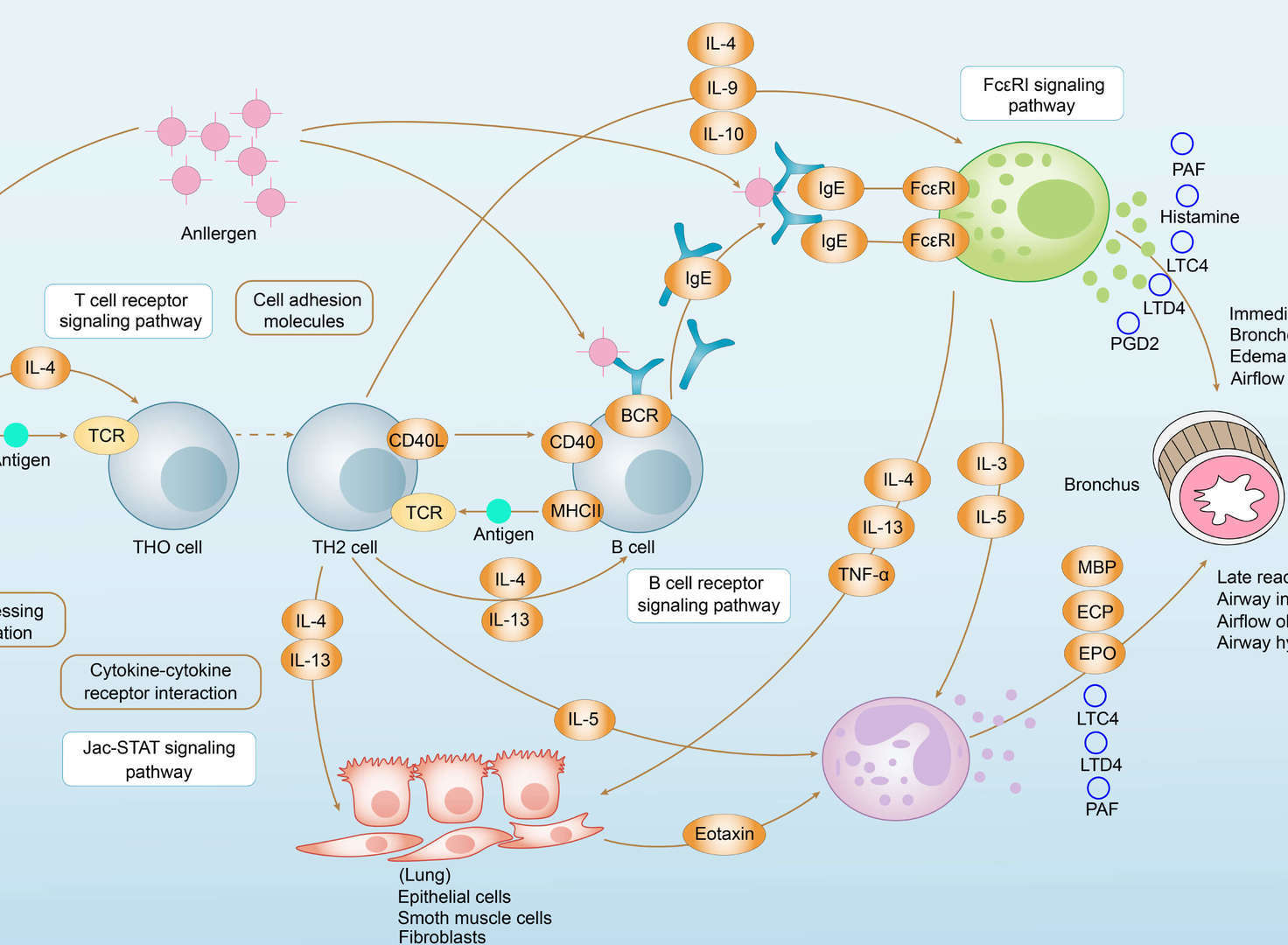 Asthma
Asthma
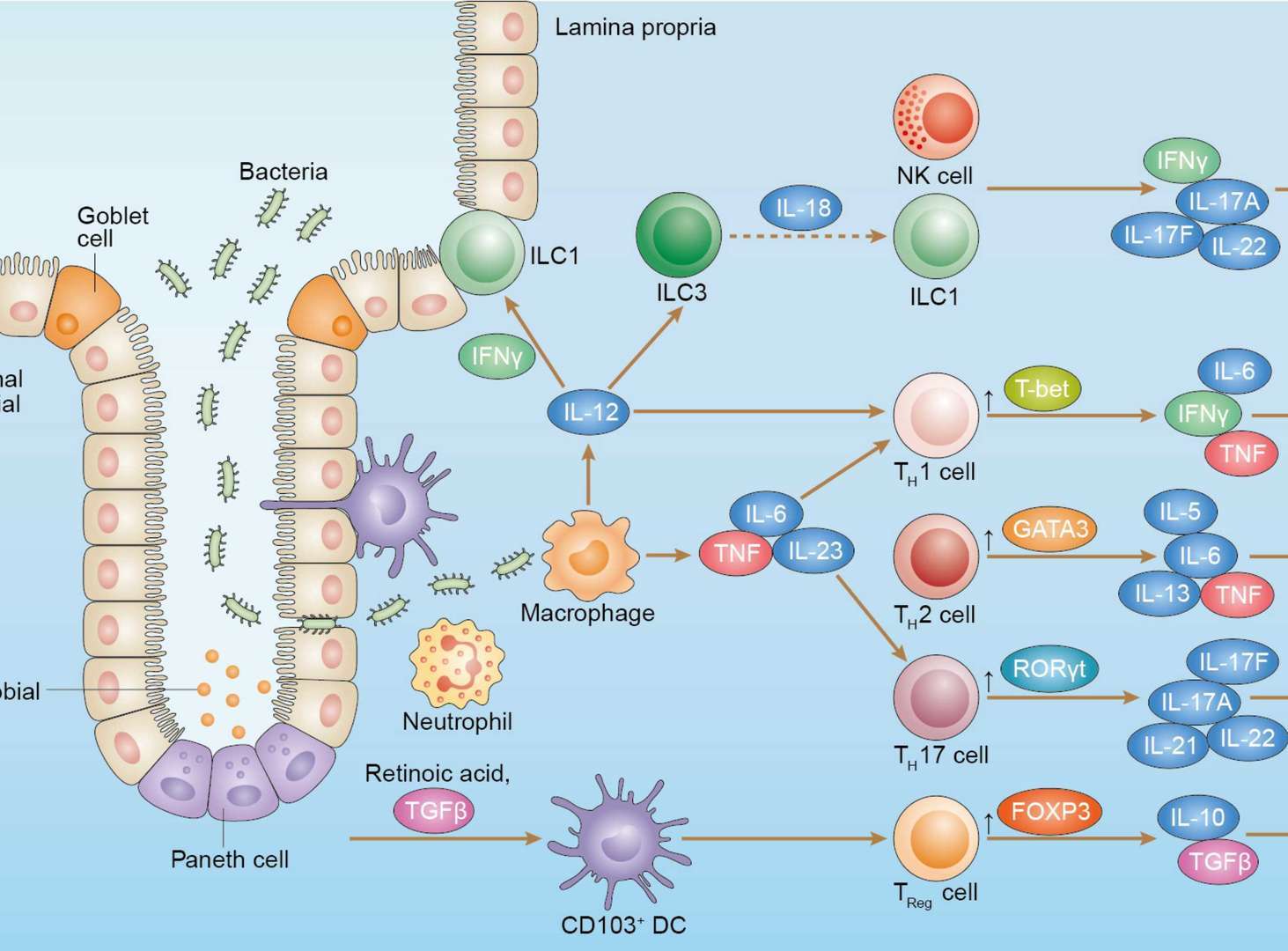 Inflammatory Bowel Diseases
Inflammatory Bowel Diseases
