Anti-Human ERBB3 Recombinant Antibody (Duligotuzumab) (TAB-H22) (CAT#: TAB-H22)
Recombinant Humanized antibody to Human ERBB3

Figure 1 Duligotuzumab vs. cetuximab in intention to treat (iTT) population showing comparable antitumor activity.
(a) Progression-free survival. (B) Overall survival.
Fayette, J., Wirth, L., Oprean, C., Udrea, A., Jimeno, A., Rischin, D.,... & O’Brien, P. (2016). Randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN Study). Frontiers in oncology, 6, 232.

Figure 2 In vitro characterization.
89Zr-MEHD7945A showed a decrease in internalization at 4 °C (right) in all cell lines. (A) 89Zr-MEHD7945A demonstrated successful blocking with cold MEHD7945A and cetuximab at 10× and 25× doses. Blocking with DL3.6b at 10× lowered the uptake of the probe; binding was sustained at 25× dose of the anti-HER3 mAb. In both AsPC-1 and BxPC-3. (B) Non-linear regression analysis determined two sets of KD and Bmax for AsPC-1 with an. (C) The KD and Bmax values for BxPC-3 (D) are within the same range as the established values in AsPC-1 with an IC50 ~ 0.37 nM. (*Denote p < 0.01, ǂdenote p < 0.05, compared to no block).
McKnight, B. N., Kuda-Wedagedara, A. N., Sevak, K. K., Abdel-Atti, D., Wiesend, W. N., Ku, A.,... & Viola-Villegas, N. T. (2018). Imaging EGFR and HER3 through 89 Zr-labeled MEHD7945A (Duligotuzumab). Scientific reports, 8(1), 9043.

Figure 3 In vivo PET imaging.
In AsPC-1 xenografts, tumor volumes-of-interest (VOI) expressed as % ID/g generated from the 89Zr-MEHD7945A PET scans exhibited uptake as early as 24 h p.i., peaking at 72 h p.i. and retained to as long as 96 h p.i. (A) 89Zr-IgG control PET scans showed minimal uptake within the tumor at all time points. (B) Similarly in BxPC-3 tumors, PET scans exhibited uptake at 24 h p.i. with a peak at 72 h p.i. (C) Non-specific tumor uptake using 89Zr-IgG in BxPC-3 xenografts showed nominal accumulation across all time points. (D) Whole body tissue distribution revealed high tumor tissue uptake of the tracer at 24 h p.i,, which plateaued at 48 h through 120 h p.i. in BxPC-3 xenografts. A competitive blocking study using unmodified MEHD7945A at 48 h p.i. displayed at least a two-fold decrease in tumor binding, indicative of the probe's specificity. (E) Of note, normal pancreas demonstrated minimal non-specific binding on all time points, suggesting that an excellent signal-to-noise contrast can be achieved.
McKnight, B. N., Kuda-Wedagedara, A. N., Sevak, K. K., Abdel-Atti, D., Wiesend, W. N., Ku, A.,... & Viola-Villegas, N. T. (2018). Imaging EGFR and HER3 through 89 Zr-labeled MEHD7945A (Duligotuzumab). Scientific reports, 8(1), 9043.

Figure 4 In vivo competitive inhibition.
In AsPC-1 xenografts, blocking with cetuximab (EGFR block) showed an almost 2-fold decrease in 89Zr-MEHD7945A uptake, whereas blocking HER3 with DL3.6b did not change probe uptake. (A) In BxPC-3 xenografts, blocking with cetuximab (EGFR block) showed a slight decrease in 89Zr-MEHD7945A, whereas blocking with DL3.6b (HER3 block) showed a statistically significant, increase in probe accumulation. (B) IHC staining in BxPC-3 tumors blocked with 25× cetuximab (25x EGFR, left), 25x DL3.6b (25x HER3, middle) or left unblocked (right) were assessed by IHC for EGFR (top) and HER3 (bottom) expression, and showed an increase in EGFR and HER3 in both blocked cohorts. (C) Tumor sections depicted for IHC are shown in 100×. Densitometry analysis of western blots on tumor lysates (n = 2) from AsPC-1 (left) and BxPC-3 (right) that were untreated, exposed to EGFR-block with cetuximab and a HER3-block with DL3.6b. (D) Densitometry is shown as a ratio of target protein/loading control.
McKnight, B. N., Kuda-Wedagedara, A. N., Sevak, K. K., Abdel-Atti, D., Wiesend, W. N., Ku, A.,... & Viola-Villegas, N. T. (2018). Imaging EGFR and HER3 through 89 Zr-labeled MEHD7945A (Duligotuzumab). Scientific reports, 8(1), 9043.

Figure 5 MEHD7945A inhibits EGFR/HER3 signaling and cell proliferation.
A, EGFR/HER3 expression profiles in multiple HNSCC and NSCLC cell lines. Six HNSCCs (UM-SCC1, UM-SCC4, UM-SCC6, UM-SCC11A, UM-SCC38, and SCC1483 cells) and five NSCLC cell lines (NCI-H226, NCI-H292, NCI-H358, NCI-H520, and A549) were cultured in relevant media. Whole-cell lysates were obtained and separated by SDS–PAGE and immunoblotted with the indicated antibodies. B, MEHD7945A inhibits growth of cells. Cells were exposed to serial concentrations of MEHD7945A for 72 hours. Thereafter, growth of tumor cells was determined by cell proliferation analysis.
Li, C., Huang, S., Armstrong, E. A., Francis, D. M., Werner, L. R., Sliwkowski, M. X.,... & Harari, P. M. (2015). Antitumor effects of MEHD7945A, a dual-specific antibody against EGFR and HER3, in combination with radiation in lung and head and neck cancers. Molecular cancer therapeutics, 14(9), 2049-2059.

Figure 6 MEHD7945A enhances radiosensitivity.
A, MEHD7945A can sensitize cells to radiation. UM-SCC6 and NCI-H226 were incubated with 5 μg/mL of cetuximab, HER3 antibody, and MEHD7945A for 4 hours, and radiated with indicated doses. Clonogenic assays were performed as described previously. Control curves were exposed to radiation without drug treatment. B, combination of MEHD7945A with radiation can increase DNA damage. UM-SCC6 and NCI-H226 cells were incubated with MEHD7945A (20 μg/mL) for 24 hours before 4 Gy radiation treatments. γ-H2AX was analyzed by flow cytometry as described in Materials and Methods at 1, 4, 8, and 24 hours following radiation. The populations of γ-H2AX–labeled cells in G1, S, and G2–M stages were gated in each bivariant cytogram and quantitated by FlowJo software. Bottom, representative cytogram obtained at 4 hours following indicated treatment. C, representative images of γ-H2AX foci in the nucleus at 4 hours following indicated treatment. Bar graph, average number of γ-H2AX foci of 100 cells; *, P ≤ 0.05 and normalized to radiation alone.
Li, C., Huang, S., Armstrong, E. A., Francis, D. M., Werner, L. R., Sliwkowski, M. X.,... & Harari, P. M. (2015). Antitumor effects of MEHD7945A, a dual-specific antibody against EGFR and HER3, in combination with radiation in lung and head and neck cancers. Molecular cancer therapeutics, 14(9), 2049-2059.

Figure 7 MEHD7945A blocked radiation-induced activation of EGFR and HER3 and redistributed cell-cycle phases.
A, UM-SCC6 and NCI-H226 cells were treated with MEHD7945A at indicated doses (pretreated 24 hours before XRT), XRT, or a combination of both. Proteins were harvested after 4 Gy radiation at the indicated times. Whole-cell lysates were obtained and separated by SDSPAGE and immunoblotted with the indicated antibodies. B, following treatment, cells were harvested and fixed 24 hours after 2 Gy or 4 Gy radiation. Following PI staining, cell cycle was assessed by flow cytometry. Data were analyzed by ModFit software.
Li, C., Huang, S., Armstrong, E. A., Francis, D. M., Werner, L. R., Sliwkowski, M. X.,... & Harari, P. M. (2015). Antitumor effects of MEHD7945A, a dual-specific antibody against EGFR and HER3, in combination with radiation in lung and head and neck cancers. Molecular cancer therapeutics, 14(9), 2049-2059.
Specifications
- Host Species
- undefined
- Derivation
- Humanized
- Type
- IgG1 - kappa
- Specificity
- ERBB3 (receptor tyrosine-protein kinase erbB-3, HER3) [Homo sapiens]
- Species Reactivity
- Human
- Applications
- ELISA, IP, FC, FuncS, Neut, IF, ICC, Activ, Block, Inhib, WB
- Generic Name
- duligotuzumab
- ATC Code
- L01X-C
- Related Disease
- Cancers
Product Property
- Purity
- >95.0%. Determined by analysis by RP-HPLC & analysis by SDS-PAGE.
Applications
- Application Notes
- The ERBB3 antibody has been reported in applications of ELISA, IP, FC, FuncS, Neut, IF, ICC, Activ, Block, Inhib, WB.
Target
- Alternative Names
- duligotuzumab;ERBB3;v-erb-b2 erythroblastic leukemia viral oncogene homolog 3 (avian);LCCS2, lethal congenital contracture syndrome 2;receptor tyrosine-protein kinase erbB-3;HER3;proto-oncogene-like protein c-ErbB-3;tyrosine kinase-type cell surface recep
- Gene ID
- 2065
- UniProt ID
- P21860
Related Resources
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Downloads
Download resources about recombinant antibody development and antibody engineering to boost your research.
See other products for "Duligotuzumab"
Afuco™ Anti-ERBB3 ADCC Recombinant Antibody (Duligotuzumab), ADCC EnhancedThis product is an ADCC enhanced antibody produced by our Afuco™ platform. Recombinant Humanized antibody to Human ERBB3
See other products for "ERBB3"
Single-domain Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NAB-1729-sdAb | Recombinant Anti-human ERBB3 VHH Single Domain Antibody | WB, ICC, ChiP, FA, ELISA | Llama VHH |
| TAB-070CT | Llama Anti-ERBB3 Recombinant Single Domain Antibody (TAB-070CT) | FC, Block | Llama VHH |
| PABC-556 | Recombinant Llama Anti-ERBB3 Single Domain Antibody (BCD090-M2) | ELISA, SPR | Llama VHH |
Recombinant Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1319z | Mouse Anti-ERBB3 Recombinant Antibody (clone 22C5) | ELISA, ICC, IF, WB | Mouse IgG1 |
| HPAB-0022-WJ | Human Anti-ERBB3 Recombinant Antibody (HPAB-0022-WJ) | ELISA, Inhib, WB, FuncS | Human IgG1, λ |
| HPAB-0024-WJ | Human Anti-ERBB3 Recombinant Antibody (HPAB-0024-WJ) | ELISA | Human IgG1 |
| HPAB-0025-WJ | Human Anti-ERBB3 Recombinant Antibody (HPAB-0025-WJ) | ELISA | Human IgG1 |
| HPAB-M0305-YC | Mouse Anti-ERBB3 Recombinant Antibody (clone 11G01) | ELISA, Neut | Mouse IgG1, κ |
Humanized Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-892 | Anti-Human ERBB3/ErbB 3 Recombinant Antibody (Seribantumab) | IF, IP, Neut, FuncS, ELISA, FC, ICC | IgG2 - lambda |
| TAB-059CT | Anti-Human HER3 Recombinant Antibody (LMAb3) | WB, ELISA, Inhibition, FC | Humanized antibody |
| TAB-057CT-F(E) | Human Anti-ERBB3 Recombinant Antibody; Fab Fragment (TAB-057CT-F(E)) | Inhibion, ELISA | Humanized Fab |
| TAB-058CT-F(E) | Human Anti-ERBB3 Recombinant Antibody; Fab Fragment (TAB-058CT-F(E)) | WB, ELISA, Inhib | Humanized Fab |
| TAB-063CT-F(E) | Human Anti-ERBB3 Recombinant Antibody; Fab Fragment (TAB-063CT-F(E)) | ELISA, Inhib, FC | Humanized Fab |
Immunotoxin
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-L074E | HRGβ2-PE immunotoxin | Cytotoxicity assay, Functional assay |
Mouse Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0508CL-S(P) | Anti-Human ERBB3 Recombinant Antibody scFv Fragment (1A5) | ELISA, Inhib, FuncS | |
| TAB-0509CL-S(P) | Anti-Human ERBB3 Recombinant Antibody scFv Fragment (3D4) | ELISA, Inhib, FuncS | |
| TAB-0508CL-F(E) | Anti-Human ERBB3 Recombinant Antibody Fab Fragment (1A5) | ELISA, Inhib, FuncS | |
| TAB-0509CL-F(E) | Anti-Human ERBB3 Recombinant Antibody Fab Fragment (3D4) | ELISA, Inhib, FuncS | |
| TAB-0552CL-F(E) | Mouse Anti-ERBB3 Recombinant Antibody; Fab Fragment (TAB-0552CL-F(E)) | ELISA, Inhib | Mouse Fab |
Chimeric Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-061CT | Human Anti-ERBB3 Recombinant Antibody (TAB-061CT) | Inhib, ELISA | Chimeric (Rabbit/Human) antibody |
| TAB-061CT-S(P) | Human Anti-ERBB3 Recombinant Antibody; scFv Fragment (TAB-061CT-S(P)) | Inhib, ELISA | Human scFv |
| TAB-061CT-F(E) | Human Anti-ERBB3 Recombinant Antibody; Fab Fragment (TAB-061CT-F(E)) | Inhib, ELISA | Chimeric (Rabbit/Human) Fab |
Human Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-065CT | Human Anti-ERBB3 Recombinant Antibody (TAB-065CT) | ELISA, FC | Human IgG1 |
| TAB-066CT | Human Anti-ERBB3 Recombinant Antibody (TAB-066CT) | ELISA, Inhibion, FC | Human IgG |
| TAB-062CT-S(P) | Human Anti-ERBB3 Recombinant Antibody; scFv Fragment (TAB-062CT-S(P)) | ELISA, Inhib, FC | Human scFv |
| TAB-065CT-S(P) | Human Anti-ERBB3 Recombinant Antibody; scFv Fragment (TAB-065CT-S(P)) | ELISA, FC | Human scFv |
| TAB-066CT-S(P) | Human Anti-ERBB3 Recombinant Antibody; scFv Fragment (TAB-066CT-S(P)) | ELISA, Inhibion, FC | Human scFv |
Neutralizing Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-738CQ | Mouse Anti-ERBB3 Recombinant Antibody (clone CBL932) | Neut, IP | Mouse IgG1 |
| NEUT-739CQ | Mouse Anti-ERBB3 Recombinant Antibody (clone CBL483) | FC, CyTOF®, ELISA, Neut | Mouse IgG1 |
| NEUT-740CQ | Human Anti-ERBB3 Recombinant Antibody (clone CBL1019) | Neut | Human IgG1, κ |
| NEUT-741CQ | Human Anti-ERBB3 Recombinant Antibody (clone CBL1020) | Neut | Human IgG1, κ |
| NEUT-742CQ | Mouse Anti-ERBB3 Recombinant Antibody (clone M908) | Neut | Mouse IgG1 |
Rabbit Monoclonal Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-1179 | Hi-Affi™ Rabbit Anti-ERBB3 Recombinant Antibody (clone DS1179AB) | ICC, IF, WB | Rabbit IgG |
Fab Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0286-YC-F(E) | Mouse Anti-ERBB3 Recombinant Antibody (clone 1153); Fab Fragment | ELISA, FuncS, FC | Mouse Fab |
| HPAB-0287-YC-F(E) | Mouse Anti-ERBB3 Recombinant Antibody (clone 920104); Fab Fragment | ELISA, FuncS, FC | Mouse Fab |
| HPAB-0288-YC-F(E) | Mouse Anti-ERBB3 Recombinant Antibody (clone 1126); Fab Fragment | ELISA, FuncS, FC | Mouse Fab |
| HPAB-0289-YC-F(E) | Mouse Anti-ERBB3 Recombinant Antibody (clone 12511); Fab Fragment | ELISA, FuncS, FC | Mouse Fab |
| HPAB-0290-YC-F(E) | Human Anti-ERBB3 Recombinant Antibody; Fab Fragment (HPAB-0290-YC-F(E)) | ELISA, FuncS, FC | Human Fab |
ADCC Enhanced Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-H21 | Afuco™ Anti-ERBB3 ADCC Recombinant Antibody (Duligotumab), ADCC Enhanced | FC, IP, ELISA, Neut, FuncS, IF | ADCC enhanced antibody |
| AFC-TAB-422CQ | Afuco™ Anti-ERBB3 ADCC Recombinant Antibody (Elgemtumab), ADCC Enhanced | ELISA, IHC, FC, IP, IF, BL | ADCC enhanced antibody |
| AFC-TAB-189 | Afuco™ Anti-ERBB3 Recombinant Antibody (AFC-TAB-189), ADCC Enhanced | IP, IF, FuncS, FC, Neut, ELISA | Human IgG1, κ |
| AFC-TAB-H22 | Afuco™ Anti-ERBB3 ADCC Recombinant Antibody (Duligotuzumab), ADCC Enhanced | ELISA, IP, FC, FuncS, Neut, IF | ADCC enhanced antibody |
| AFC-TAB-892 | Afuco™ Anti-ERBB3 ADCC Recombinant Antibody (Seribantumab), ADCC Enhanced | IF, IP, Neut, FuncS, ELISA, FC | ADCC enhanced antibody |
scFv Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-1136-FY-F(E) | Human Anti-ERBB3 Recombinant Antibody; Fab Fragment (HPAB-1136-FY-F(E)) | ELISA, FC | Human Fab |
| HPAB-1137-FY-F(E) | Human Anti-ERBB3 Recombinant Antibody; Fab Fragment (HPAB-1137-FY-F(E)) | ELISA, FC | Human Fab |
| HPAB-1138-FY-F(E) | Human Anti-ERBB3 Recombinant Antibody (clone H3); scFv Fragment | ELISA | Human scFv |
| HPAB-1139-FY-F(E) | Human Anti-ERBB3 Recombinant Antibody (clone A5); scFv Fragment | ELISA | Human scFv |
| HPAB-1140-FY-F(E) | Human Anti-ERBB3 Recombinant Antibody (clone B12); scFv Fragment | ELISA | Human scFv |
Customer Reviews and Q&As
There are currently no Customer reviews or questions for TAB-H22. Click the button above to contact us or submit your feedback about this product.
View the frequently asked questions answered by Creative Biolabs Support.
For Research Use Only. Not For Clinical Use.
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.















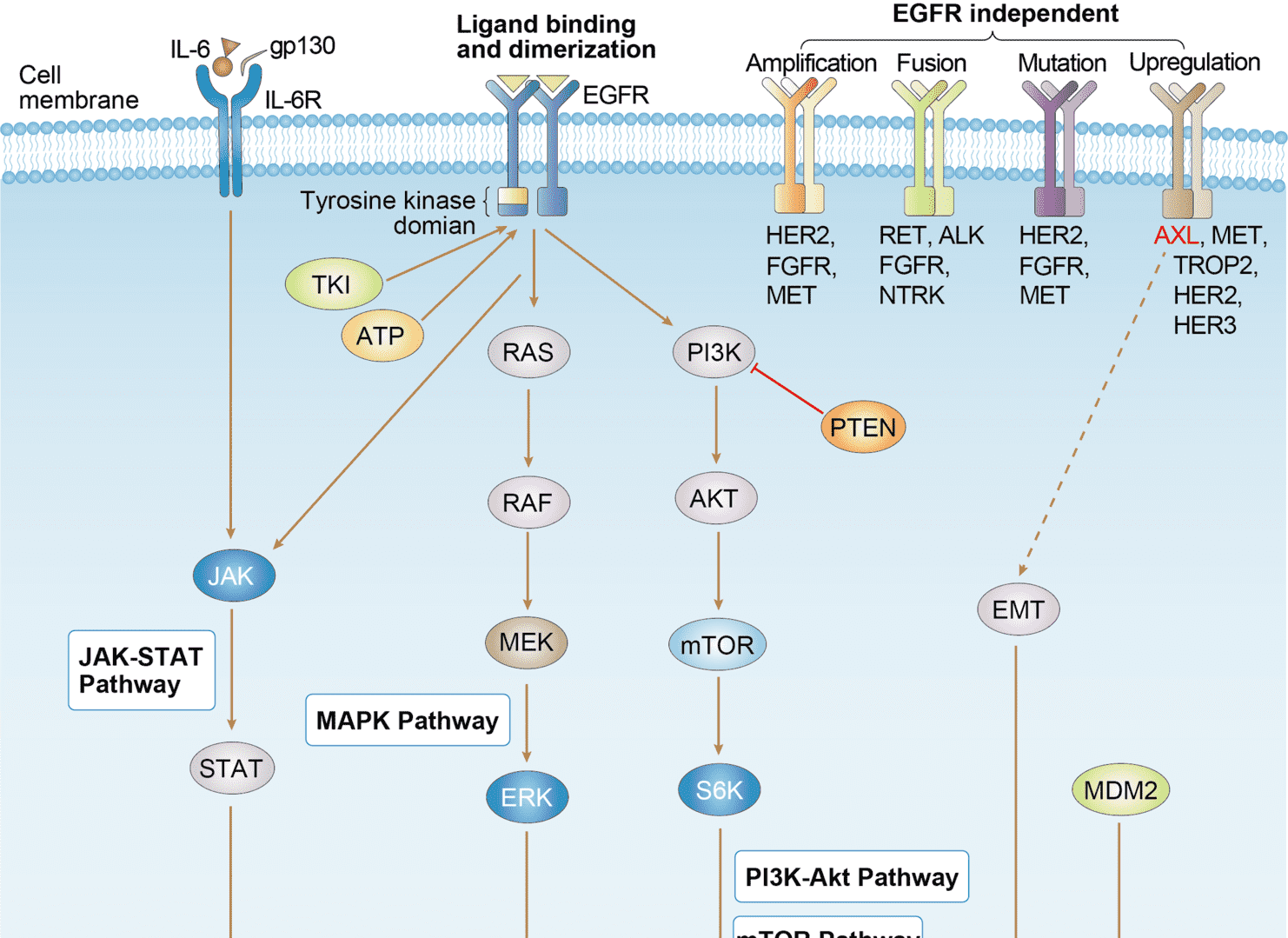 EGFR Tyrosine Kinase Inhibitor Resistance
EGFR Tyrosine Kinase Inhibitor Resistance
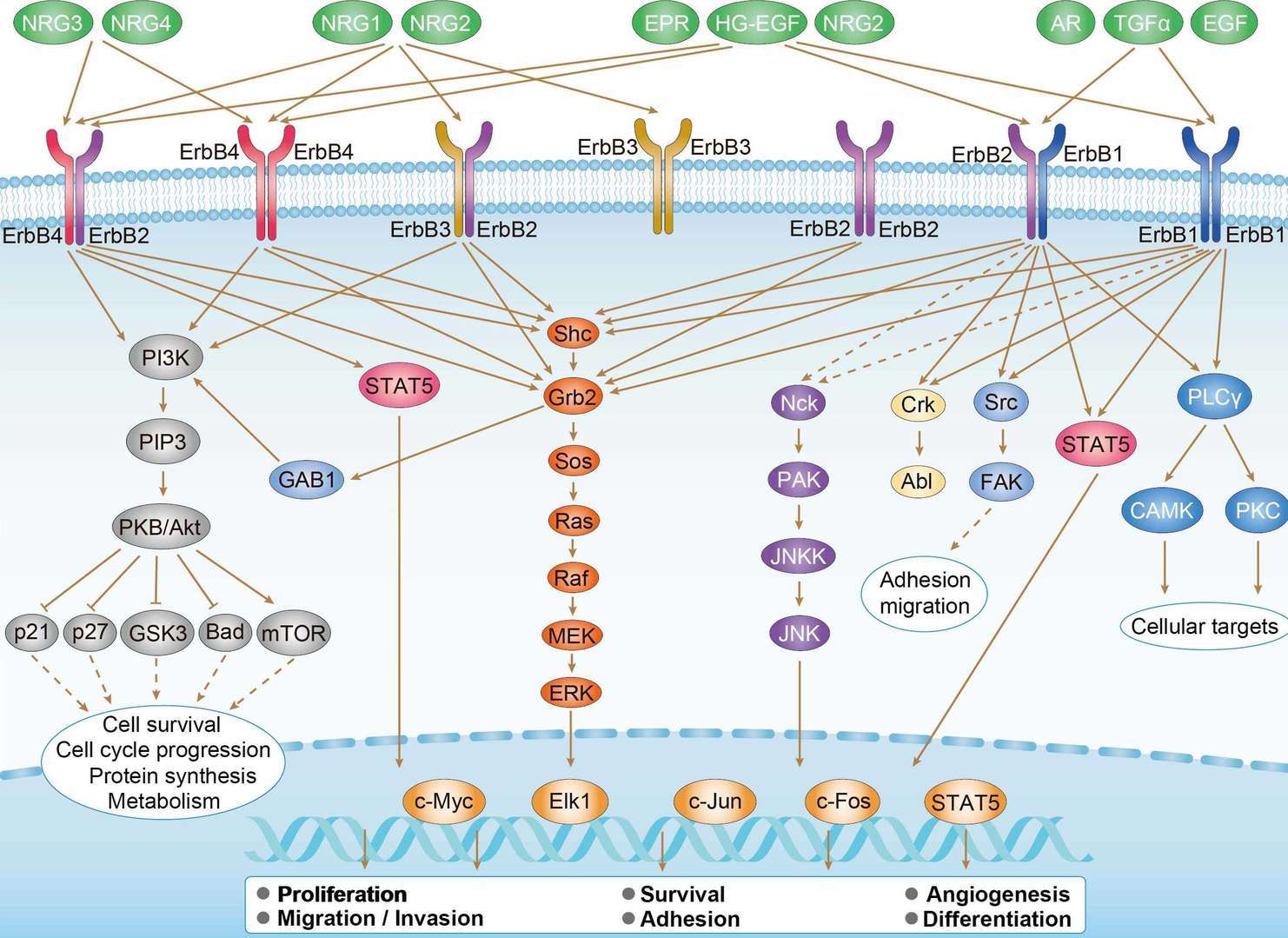 ErbB Signaling Pathway
ErbB Signaling Pathway
