Anti-Human IL5 Recombinant Antibody (TAB-031)
CAT#: TAB-031
Recombinant monoclonal antibody to Human IL5. It is a humanized monoclonal antibody that recognizes interleukin-5 (IL-5), and is used to treat certain kinds of asthma and white blood cell diseases. Recent studies have concluded that mepolizumab may improve exacerbations in patients with severe eosinophilic asthma, an adult-onset asthma which represents less than 5% of all asthma. IL-5 is a chemical messenger in the immune system that stimulates the growth of eosinophils. In eosinophilic asthma, eosinophils are present in the lungs. When mepolizumab was given to people with eosinophilic asthma, it eliminated eosinophils from the bloodstream,and reduced eosinophils in the lungs and bone marrow.


Specifications
- Immunogen
- Recombinant human IL5.
- Host Species
- Mouse
- Derivation
- Humanized (from mouse)
- Type
- IgG1 - kappa
- Specificity
- Tested positive against native human antigen
- Species Reactivity
- Human
- Applications
- IF, IP, Neut, FuncS, ELISA, FC, ICC
- Related Disease
- Asthma
Product Property
- Purity
- >97%, by SDS-PAGE under reducing conditions and visualized by silver stain.
- Storage
- Store at -20°C for long-term storage. Store at 2-8°C for up to one month. Avoid freeze/thaw cycles.
Applications
- Application Notes
- The IL5 antibody has been reported in applications of Activ, IHC.
Target
Customer Review
There are currently no Customer reviews or questions for TAB-031. Click the button above to contact us or submit your feedback about this product.
 Jessica Green
Jessica Green Michael Evans
Michael Evans Sarah Davis
Sarah DavisQ&As
-
Is the anti-Human IL5 antibody suitable for use in Western blotting?
A: Yes, the anti-Human IL5 antibody (TAB-031) is suitable for use in Western blotting. It provides specific binding to IL5, allowing for reliable detection in Western blot assays.
-
What are the storage recommendations for the anti-Human IL5 antibody ?
A: The recommended storage condition for the anti-Human IL5 antibody (TAB-031) is at -20°C or lower. For short-term storage, it can be kept at 2-8°C. To ensure stability, avoid repeated freeze-thaw cycles.
-
Can the anti-Human IL5 antibody be used in immunoprecipitation assays?
A: Yes, the anti-Human IL5 antibody (TAB-031) can be used in immunoprecipitation assays. It provides specific binding to IL5, enabling the successful precipitation of the target protein from complex mixtures.
-
Is the anti-Human IL5 antibody effective in ELISA applications?
A: Yes, the anti-Human IL5 antibody (TAB-031) is effective in ELISA applications. It has been validated for use in such assays and provides reliable detection of IL5.
-
What is the optimal dilution for using the anti-Human IL5 antibody in immunofluorescence?
A: The optimal dilution for using the anti-Human IL5 antibody (TAB-031) in immunofluorescence is typically 1:100 to 1:500. It is advisable to perform a dilution series to determine the best working concentration for your specific experimental conditions.
View the frequently asked questions answered by Creative Biolabs Support.
Citations
-
Calzetta, Luigino, et al. "Targeting IL-5 pathway against airway hyperresponsiveness: A comparison between benralizumab and mepolizumab." British Journal of Pharmacology 177.20 (2020): 4750-4765. https://doi.org/10.1111/bph.15240This study investigates the effectiveness of targeting the IL-5 pathway in addressing airway hyperresponsiveness (AHR) in asthma by comparing two monoclonal antibodies, benralizumab and mepolizumab. The research focuses on their ability to modulate AHR in human airways passively sensitized to mimic asthmatic conditions. The study measures the inhibition of AHR to histamine, parasympathetic activation, and mechanical stress, as well as the modulation of cyclic AMP (cAMP) levels. The results demonstrate that both benralizumab and mepolizumab significantly reduce AHR, with benralizumab being more potent. The study concludes that targeting the IL-5/IL-5Rα axis is an effective strategy for preventing AHR.
Creative Biolabs provided the benralizumab (Cat# TAB-222) and mepolizumab (Cat# TAB-031) used in this study. These antibodies were essential for the comparative analysis of their effects on AHR and cAMP levels in human bronchial tissues. The use of these reagents allowed the researchers to determine the differential potency and efficacy of benralizumab and mepolizumab in modulating AHR, thus contributing significantly to the understanding of their therapeutic potential in treating asthma.
Cite This Product
To accurately reference this product in your publication, please use the following citation information:
(Creative Biolabs Cat# TAB-031, RRID: AB_3111765)
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Biosimilar Overview
Please refer to Mepolizumab Overview to learn more about the mechanism of action, clinical projects, and approved drugs of Mepolizumab.
Related Diseases
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
Protocol & Troubleshooting
We have outlined the assay protocols, covering reagents, solutions, procedures, and troubleshooting tips for common issues in order to better assist clients in conducting experiments with our products. View the full list of Protocol & Troubleshooting.
See other products for "IL5"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1202z | Mouse Anti-IL5 Recombinant Antibody (clone 37C3) | WB, IP, IF, ELISA | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-136 | Anti-Human IL5 Recombinant Antibody (TAB-136) | Neut, ELISA, IF, IP, FuncS, FC, IHC | IgG4 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-0631CT | Recombinant Mouse anti-Human IL5 Monoclonal antibody (RT-6) | ELISA, ELISPOT, IHC-Fr, Neut, WB |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1437CQ | Mouse Anti-IL5 Recombinant Antibody (clone CBL632) | ELISA, Neut | Mouse IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1438CQ | Mouse Anti-IL5 Recombinant Antibody (clone CBL420) | Neut | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1439CQ | Rat Anti-IL5 Recombinant Antibody (clone CBL900) | ELISA, FC, IF, Neut, WB | Rat IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1440CQ | Rat Anti-IL5 Recombinant Antibody (clone JES1-39D10) | ELISA, ELISPOT, Neut, ICFC, IHC, WB | Rat IgG2a, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1441CQ | Rat Anti-IL5 Recombinant Antibody (clone JES1-5A10) | ELISA, ELISPOT, Neut | Rat IgG2a, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1443CQ | Rat Anti-IL5 Recombinant Antibody (clone TRFK4) | ELISA, Inhib, WB | Rat IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0001-CN | Human Anti-IL5 Recombinant Antibody (clone h39D10) | ELISA | Humanized IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0001-CN-S(P) | Human Anti-IL5 Recombinant Antibody (clone h39D10); scFv Fragment | ELISA | Humanized scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0001-CN-F(E) | Human Anti-IL5 Recombinant Antibody (clone h39D10); Fab Fragment | ELISA | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-N0359-YC | Human Anti-IL5 Recombinant Antibody (HPAB-N0359-YC) | ELISA, Inhib | Human IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-N0359-YC-S(P) | Human Anti-IL5 Recombinant Antibody; scFv Fragment (HPAB-N0359-YC-S(P)) | ELISA, Inhib | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-N0359-YC-F(E) | Human Anti-IL5 Recombinant Antibody; Fab Fragment (HPAB-N0359-YC-F(E)) | ELISA, Inhib | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0250CQ | Human Anti-IL5 Recombinant Antibody (HPAB-0250CQ) | ELISA, Neut, FuncS | Human IgG4, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0250CQ-F(E) | Human Anti-IL5 Recombinant Antibody; Fab Fragment (HPAB-0250CQ-F(E)) | ELISA, Neut, FuncS | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0250CQ-S(P) | Human Anti-IL5 Recombinant Antibody; scFv Fragment (HPAB-0250CQ-S(P)) | ELISA, Neut, FuncS | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-031 | Afuco™ Anti-IL5 ADCC Recombinant Antibody ADCC Enhanced (AFC-TAB-031) | IF, IP, Neut, FuncS, ELISA, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-136 | Afuco™ Anti-IL5 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-136) | Neut, ELISA, IF, IP, FuncS, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0053-YJ-S(P) | Human Anti-IL5 Recombinant Antibody (clone CMX5-1); scFv Fragment | ELISA | Humanized scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0053-YJ-F(E) | Human Anti-IL5 Recombinant Antibody (clone CMX5-1); Fab Fragment | ELISA | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0875-FY-F(E) | Human Anti-IL5 Recombinant Antibody; Fab Fragment (HPAB-0875-FY-F(E)) | ELISA | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0875-FY-S(P) | Human Anti-IL5 Recombinant Antibody; scFv Fragment (HPAB-0875-FY-S(P)) | ELISA | Humanized scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY144 | CytoStream™ Rat Anti-IL5 Recombinant Antibody (VS-0225-XY144) | FC | Rat IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY145 | CytoStream™ Rat Anti-IL5 Recombinant Antibody (clone TRFK5) | FC | Rat IgG1, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0325-FY206 | Human Anti-IL5 (clone NOL48-1-D5) scFv-Fc Chimera | Inhib, ELISA | Human IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0625-YC192 | Recombinant Anti-IL5 Eliminating Antibody, pH-Sensitive (VS-0625-YC192) | Antigen-Sweeping In Vivo. | Rat IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC204 | SmartAb™ Recombinant Anti-IL5 pH-dependent Antibody (VS-0825-YC204) | IF, IP, Neut, ELISA, FC, ICC | Human IgG1 kappa |
Popular Products

Application: Neut, ELISA, IF, IP, FuncS, FC, IHC

Application: WB, FC, IP, ELISA, Neut, FuncS, IF

Application: IF, IP, Neut, FuncS, ELISA, FC, WB

Application: ELISA, Neut, IF, IP, FC, FuncS

Application: ELISA, FC, IP, FuncS, IF, Neut, ICC

Application: WB, FuncS, IF, Neut, ELISA, FC, IP

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: WB, FC, IP, ELISA, Neut, FuncS, IF

Application: IF, IP, Neut, FuncS, ELISA, FC, ICC

Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: WB, Neut, FuncS
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.




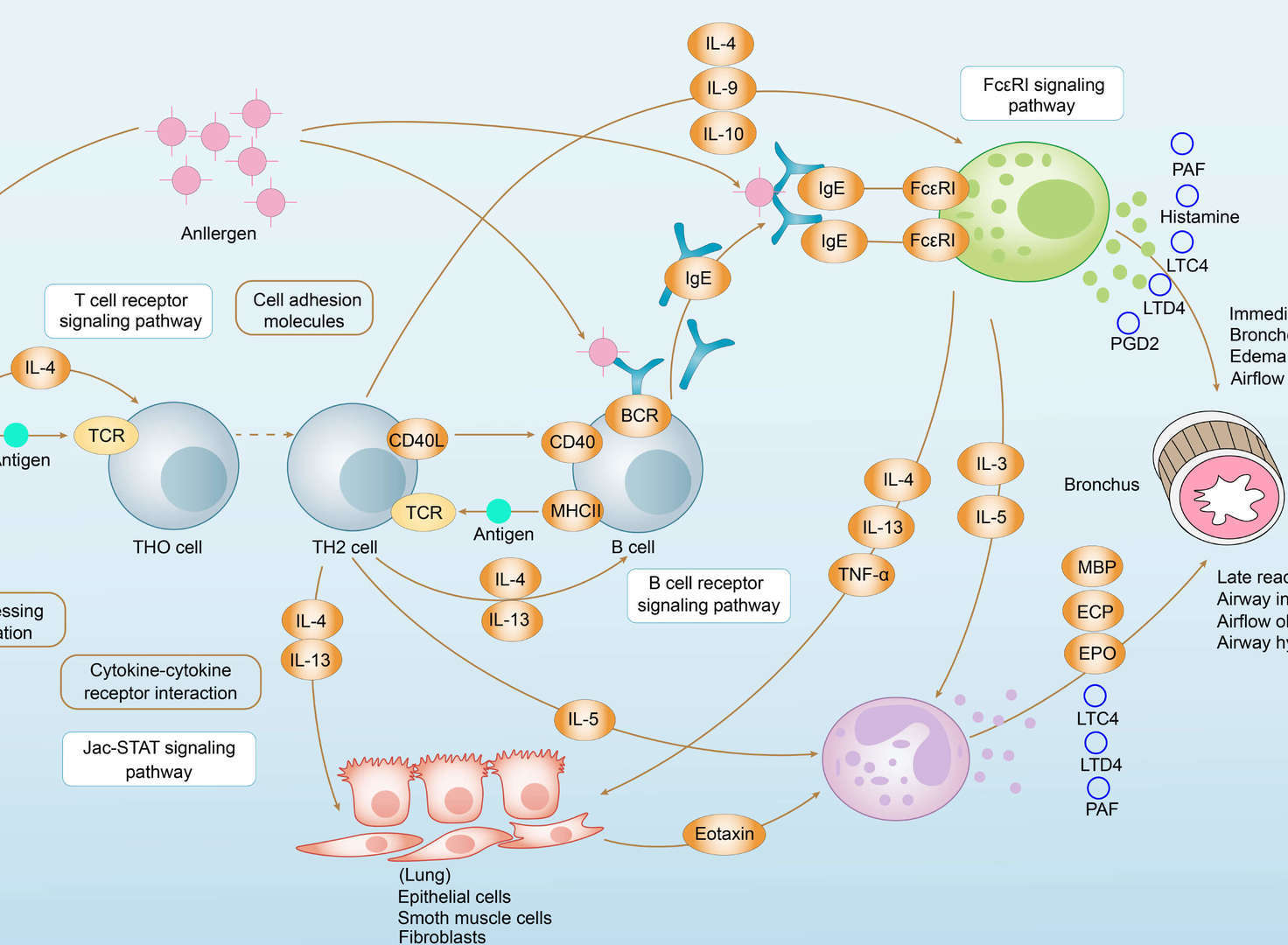 Asthma
Asthma
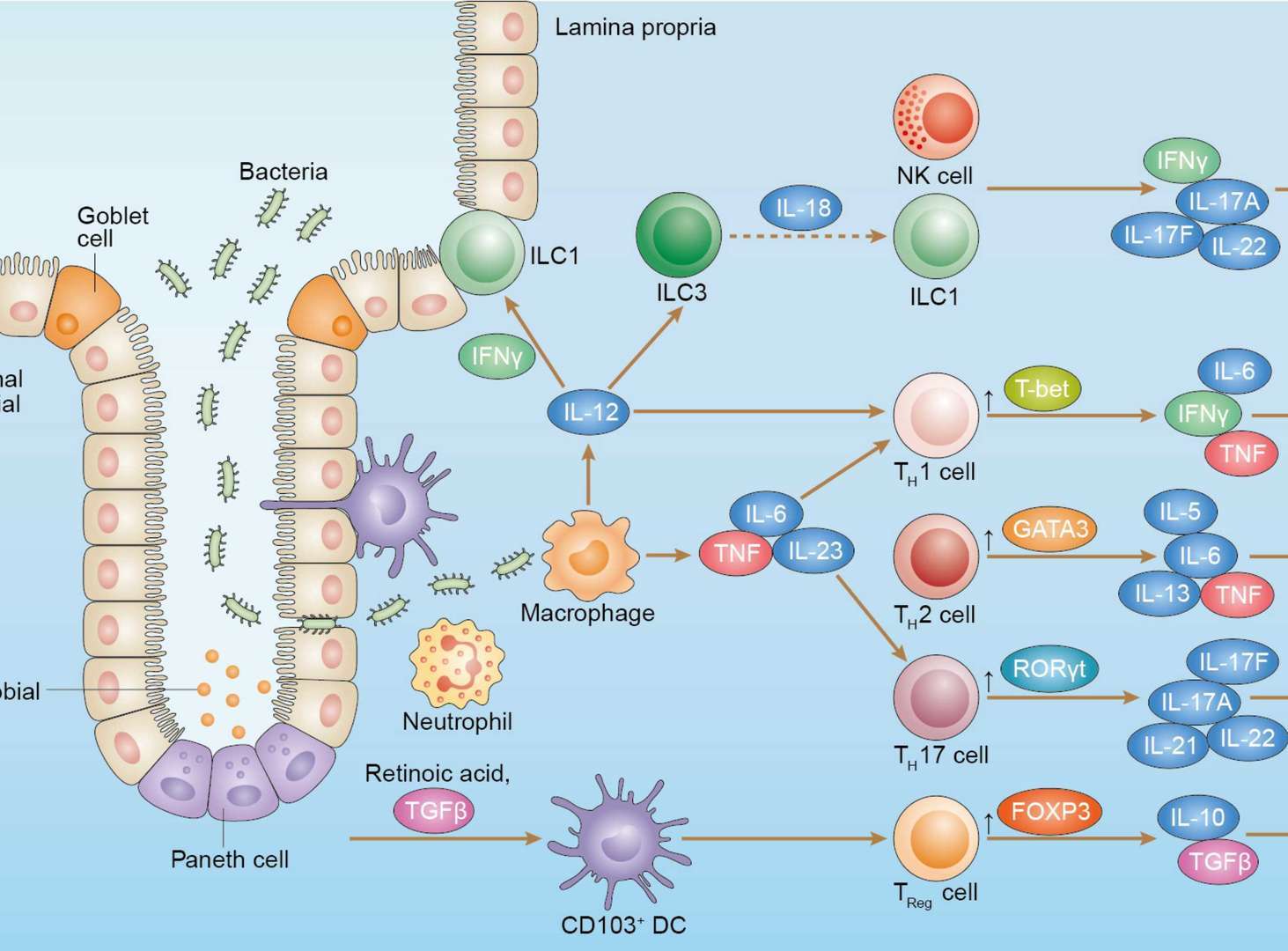 Inflammatory Bowel Diseases
Inflammatory Bowel Diseases
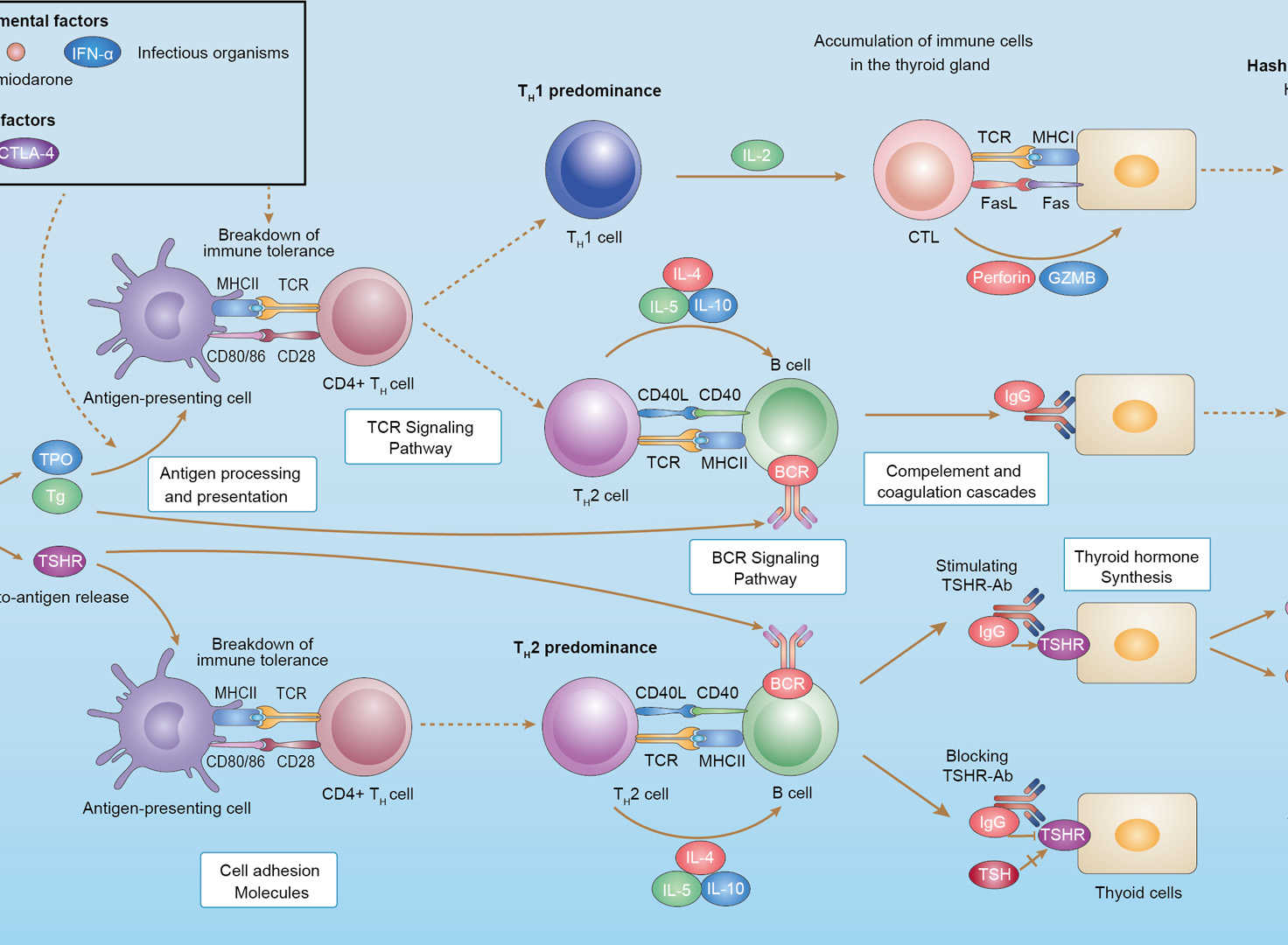 Autoimmune Thyroid Disease
Autoimmune Thyroid Disease











