Evolocumab Overview
Introduction of Evolocumab
Evolocumab is a fully human monoclonal antibody (IgG2λ type) designed for the treatment of hyperlipidemia. It targets the proprotein convertase subtilisin/kexin type 9 (PCSK9). Evolocumab has been successively approved for marketing in USA, European Union, Canada, Australia, and Japan.
Mechanism of Action of Evolocumab
Evolocumab recognizes and attaches to the enzyme PCSK9. This enzyme attaches to cholesterol receptors on the surface of liver cells and causes these receptors to be absorbed and broken down inside the cells. By attaching and blocking PCSK9, Evolocumab prevents the receptors from being broken down and therefore increases their numbers on the cell surface, where they can attach to low-density lipoprotein-cholesterol (LDL-C) and remove it from the bloodstream. This helps to reduce the amount of cholesterol in the blood. Evolocumab also helps to reduce other fatty substances from blood in patients with mixed dyslipidemia.
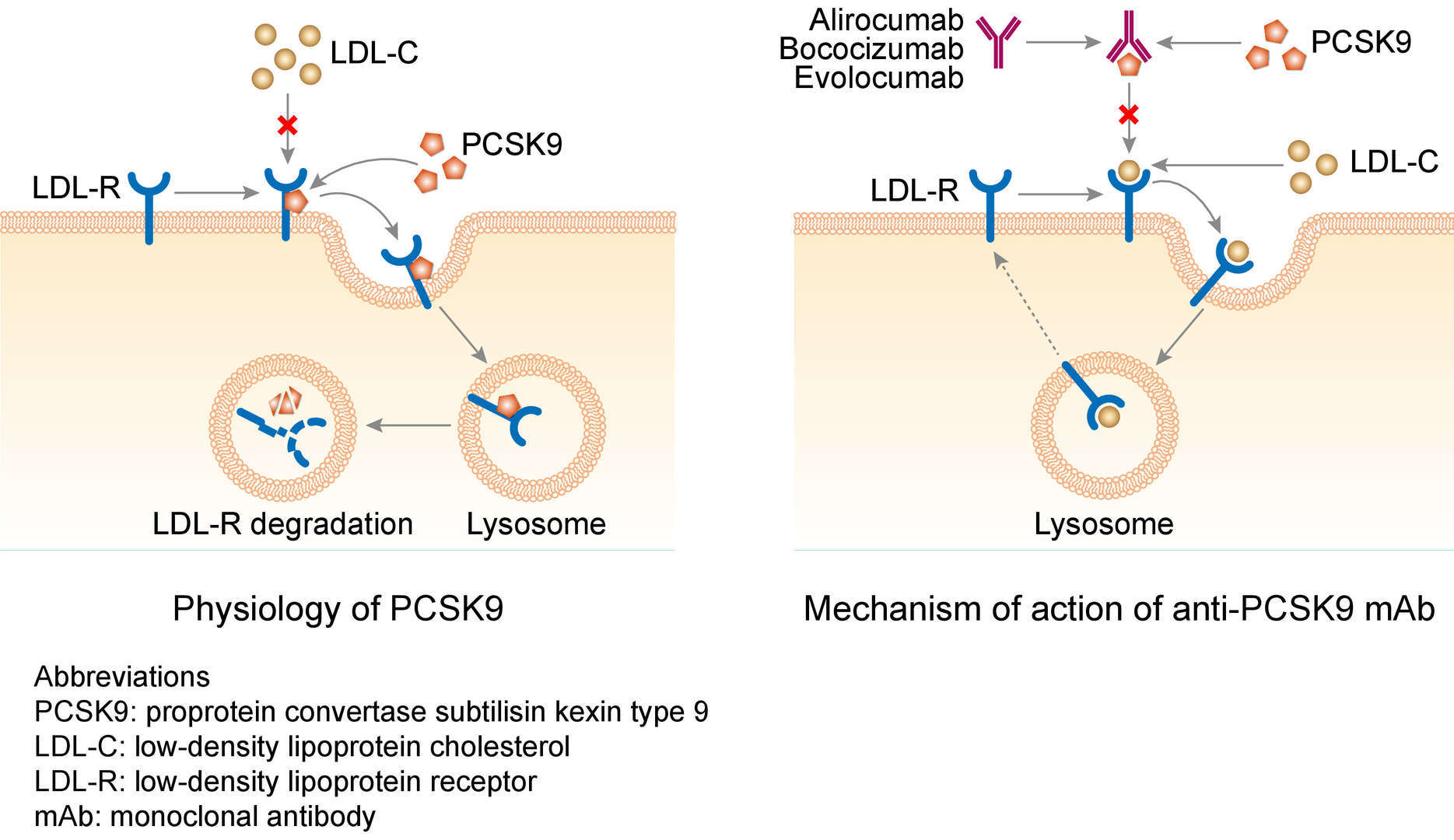 Fig.1 Mechanism of action of evolocumab
Fig.1 Mechanism of action of evolocumab
Clinical Projects of Evolocumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03480568 | Not yet recruiting | Hemodialysis, Peritoneal Dialysis, Hypercholesterolemia, Atherosclerotic Disease | Baylor Research Institute | March 29, 2018 |
| NCT03533959 | Recruiting | Coronary Artery Disease Progression | Kobe University | May 23, 2018 |
| NCT02938949 | Recruiting | Myocardial Infarction, Hypercholesterolemia | Virginia Commonwealth University | October 19, 2016 |
| NCT03529253 | Recruiting | Coronary Artery Disease, Angina Pectoris | Kobe University | May 18, 2018 |
| NCT03014830 | Active, not recruiting | Atherosclerosis, Coronary Heart Disease | Washington University School of Medicine | January 9, 2017 |
| NCT03379558 | Recruiting | Hypercholesterolemia | Sanofi | December 20, 2017 |
| NCT03542110 | Not yet recruiting | Saphenous Vein Graft Atherosclerosis | Minneapolis Heart Institute Foundation | May 31, 2018 |
| NCT03344692 | Not yet recruiting | Type2 Diabetes | Nantes University Hospital | November 17, 2017 |
| NCT02959047 | Recruiting | Peripheral Arterial Disease | University of Virginia | November 8, 2016 |
| NCT03004001 | Recruiting | Nephrotic Syndrome | Gloria Vega | December 28, 2016 |
| NCT03067844 | Recruiting | Coronary Vessel, Coronary Circulation, Myocardial Atheroma | University Hospital Inselspital, Berne | March 1, 2017 |
| NCT02992301 | Recruiting | Atherosclerosis, Hyperlipidemia | Westside Medical Associates of Los Angeles | December 14, 2016 |
| NCT03510715 | Not yet recruiting | Hypercholesterolemia | Sanofi | April 27, 2018 |
| NCT03510884 | Recruiting | Hypercholesterolemia | Sanofi | April 27, 2018 |
| NCT02715726 | Active, not recruiting | Hypercholesterolemia | Sanofi | March 22, 2016 |
| NCT02890992 | Recruiting | Hypercholesterolemia | Sanofi | September 7, 2016 |
| NCT02984982 | Active, not recruiting | Hypercholesterolemia, Acute Coronary Syndrome | Sanofi | December 7, 2016 |
| NCT02476006 | Active, not recruiting | Hypercholesterolemia | Sanofi | June 19, 2015 |
| NCT02957682 | Active, not recruiting | Hypercholesterolemia | Regeneron Pharmaceuticals | November 8, 2016 |
| NCT03273972 | Recruiting | Atherosclerosis, Cardiovascular Diseases | Cambridge University Hospitals NHS Foundation Trust | September 6, 2017 |
| NCT03355027 | Recruiting | Atherosclerosis, Cardiovascular Diseases | Cambridge University Hospitals NHS Foundation Trust | November 28, 2017 |
| NCT03156621 | Recruiting | Homozygous Familial Hypercholesterolemia | Regeneron Pharmaceuticals | May 17, 2017 |
| NCT03415178 | Active, not recruiting | Hypercholesterolemia | Sanofi | January 30, 2018 |
| NCT03507374 | Not yet recruiting | Stroke, Intracranial Atherosclerosis, Intraplaque Hemorrhage | University of Utah | April 25, 2018 |
Approved Drugs of Evolocumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Repatha | Dyslipidemias, Hypercholesterolemia | Injection, Solution | 140 mg/mL | Subcutaneous | Amgen Europe B.V. | July 17, 2015 |

|
| Repatha | Heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease | Injection, Solution | 140 mg/mL | Subcutaneous | Amgen Inc. | August 27, 2015 |

|
| Repatha | Cardiovascular Events, Primary Hyperlipidemia, Homozygous Familial Hypercholesterolemia, Geriatrics, Pediatrics | Injection, Solution | 140 mg/mL | Subcutaneous | Amgen Canada Inc. | September 28, 2015 |

|
| Repatha | Heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease | Injection, Solution | 120/140 mg/mL | Subcutaneous | Amgen Australia Pty Ltd. | December 9, 2015 |

|
| Repatha | Hypercholesterolemia | Injection, Solution | 140 mg/mL | Subcutaneous | Amgen Astellas BioPharma K.K. | January 22, 2016 |

|
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Evolocumab
** Information presented in the table were collected from the following websites:
http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/2189401G1
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=93132
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125522
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003766/human_med_001890.jsp
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=231151
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



