Infliximab Biosimilar Pipeline Development Service
Comprehensive Solutions for Infliximab Biosimilar Development
At Creative Biolabs, we specialize in providing comprehensive development services for Infliximab biosimilars, leveraging our expertise in recombinant antibody technology and biosimilar manufacturing. Our services cover the entire biosimilar pipeline, from early-stage development and analytical characterization to large-scale production, ensuring that your Infliximab biosimilar is of the highest quality and fully compliant with global regulatory standards.
Our approach begins with in-depth structural and functional analysis of the reference product, ensuring that all critical attributes are captured and reproduced. We then proceed with cell line development, optimization of expression systems, and rigorous quality control throughout the manufacturing process. Alongside these, we provide comprehensive analytical testing to confirm biosimilarity, covering aspects like glycosylation patterns, potency, and purity.
Creative Biolabs is committed to delivering efficient and effective solutions for Infliximab biosimilar development, enabling you to navigate the complex regulatory landscape and accelerate time-to-market while maintaining consistency, quality, and compliance at every step.
- At a glance of Infliximab
Basic Information
Drug Type: Monoclonal antibody
Synonyms: Avakine; CenTNF; Infliximab (Genetical Recombination)
Target: TNF-α
Action: Inhibitors
Mechanism: TNF-α inhibitors(Tumor necrosis factor α inhibitors)
Therapeutic Areas: Immune System Diseases; Infectious Diseases; Cardiovascular Diseases
Active Indication: Mucocutaneous Lymph Node Syndrome; Behcet's uveitis; Erythrodermic psoriasis
Inactive Indication: Axial Spondyloarthritis; Berylliosis; Cachexia
Originator Organization: Janssen Global Services LLC
First Approval Date: United States (24 Aug 1998), Crohn Disease
Most Recent Events
| Date | Events |
| 2025-06-15 | Infliximab biosimilars receive expanded indication for the treatment of autoimmune hepatitis in Europe. |
| 2025-03-22 | New study shows that Infliximab significantly improves outcomes in Crohn's disease patients with refractory symptoms. |
| 2024-12-10 | FDA approves a new formulation of Infliximab for subcutaneous administration, enhancing convenience for patients. |
| 2024-08-05 | Phase IV study results demonstrate that Infliximab is effective in treating pediatric patients with severe ulcerative colitis. |
| 2024-04-18 | Infliximab biosimilars show high comparability in a head-to-head study with the reference product in patients with rheumatoid arthritis. |
- Pipeline Status
Our Infliximab Biosimilar Pipeline Offers
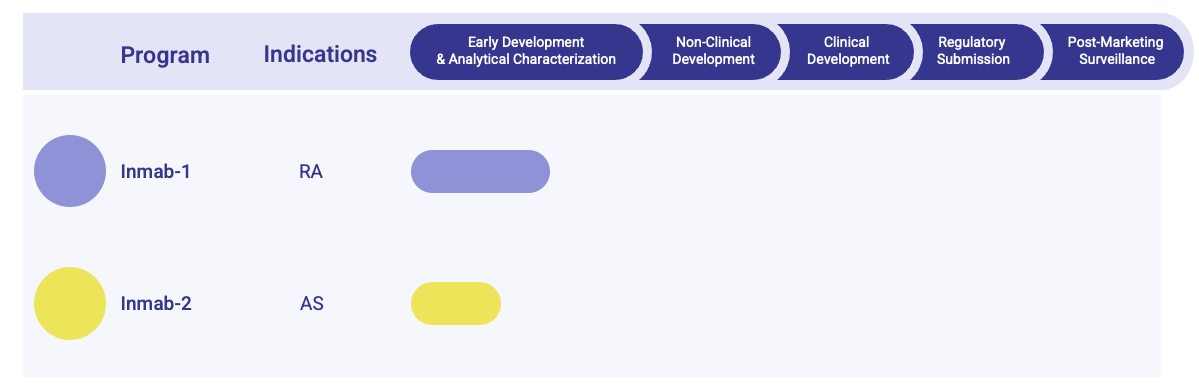
Development Workflow of Infliximab Biosimilar Pipeline
| Early Development & Analytical Characterization | ||
| 1 | Sequence Analysis and Structural Characterization | Analyzing the reference Infliximab to determine its amino acid sequence, structure, and post-translational modifications. |
| 2 | Cell Line Development | Generation of high-yield stable cell lines (typically CHO or HEK293) to produce the biosimilar. |
| 3 | Expression System Optimization | Selection of the most efficient expression system to ensure high yield and consistency in biosimilar production. |
| 4 | Analytical Testing | Conducting tests for purity, identity, and potency using techniques like HPLC, mass spectrometry, and ELISA to verify the biosimilar's quality. |
| Non-Clinical Development | ||
| 5 | In Vitro Biological Activity Testing |
|
| 6 | In Vivo Pharmacology & Toxicology |
Animal model studies to assess pharmacokinetics (PK), pharmacodynamics (PD), and safety, including:
|
| 7 | Immunogenicity Testing | Evaluating the immune response to the biosimilar, including ADA formation. |
- Available Packages
At Creative Biolabs, we offer a variety of service packages to support your Infliximab biosimilar development needs. From early development through regulatory submission, our packages are designed to ensure your biosimilar progresses smoothly at every stage.
| NO. | Item Name | Includes | Deliverables |
| 1 | Early Development & Analytical Characterization Package |
|
|
| 2 | Non-Clinical Development Package |
|
|
| 3 | Full Biosimilar Development Package |
|
|
- Why Choose Us
At Creative Biolabs, we specialize in providing comprehensive development services for Infliximab biosimilars. Here's why we are the preferred partner for your biosimilar development needs:
Extensive Expertise in Biosimilar Development
With deep knowledge and experience in recombinant antibody technologies, we provide cutting-edge solutions for Infliximab biosimilar development at every stage of the pipeline.
End-to-End Services
Our services span across early development, non-clinical testing, ensuring a smooth transition between all stages of development.
State-of-the-Art Technology
Our high-performance expression systems, cell line development, and analytical techniques ensure your Infliximab biosimilar meets the highest standards for quality, potency, and regulatory compliance.
By choosing Creative Biolabs, you gain a trusted partner with a proven track record in the successful development of Infliximab biosimilars, ensuring your product's smooth market entry and regulatory compliance.
- Ready to Advance Your Infliximab Biosimilar Program
If you're ready to start your Infliximab biosimilar development or have any questions about our services, we're here to help. Contact us today to discuss how we can assist you in bringing your product to market.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



