Muromonab-CD3 Overview
Introduction of Muromonab-CD3
Muromonab-CD3 is a recombinant murine (mouse) monoclonal antibody to human CD3. More specifically it is 93% monomeric immune globulin G type 2a (IgG2a) and directed against the CD3 (T3) receptor on the surface of human T-cells (T-lymphocytes) cultured using the murine ascites method. Muromonab-CD3 was approved for use in treating acute rejection after renal transplantation in 1997 which was the first monoclonal antibody to be approved for clinical use in humans and its indications were later broadened to include rejection after heart and liver transplantation. However, it is a potent immunosuppressive agent and may result in reactivation of hepatitis B in susceptible patients. Furthermore, the initial engagement of CD3 receptors can result in a transient, acute release of proinflammatory cytokines (cytokine release syndrome) with symptoms of high fever, weakness, dyspnea, nausea, chest pain and diarrhea arising within the first two days of starting therapy.
Mechanism of Action of Muromonab-CD3
Transplantation of solid organs has emerged as a viable therapeutic modality for the treatment of a variety of ailments, such as end stage renal disease. Rejection of solid organ allografts is the result of a complex series of interactions involving coordination between both the innate and adaptive immune system with T cells central to this process. The ability of recipient T cells to recognize donor-derived antigens, called allorecognition, initiates allograft rejection. Once recipient T cells become activated, they undergo clonal expansion, differentiate into effector cells, and migrate into the graft where they promote tissue destruction. Muromonab-CD3 is the first anti-CD3 monoclonal antibody (mAb) available for treatment in humans. It is a murine mAb of the IgG-2a class, directed against the CD3 molecule which is present on the surface of human thymocytes and mature T cells. The CD3 molecule is closely associated with the T cell antigen receptor (TCR) in the so-called CD3/TCR complex. This complex plays a vital role in T cell function: antigen recognition by the TCR results in transmembrane signal transduction via the CD3 molecule and subsequent T cell proliferation and activation of cytotoxic cells. Muromonab-CD3 binds to the T cell receptor-CD3-complex (specifically the CD3 epsilon chain) on the surface of circulating T cells, initially leading to an activation, but subsequently inducing blockage and apoptosis of the T cells. This protects the transplant against the T cells. It also has some adverse effects. Especially during the first infusion, the binding of muromonab-CD3 to CD3 can activate T cells to release cytokines like tumor necrosis factor and interferon gamma. This cytokine release syndrome, or CRS, includes side effects like skin reactions, fatigue, fever, chills, myalgia, headaches, nausea and diarrhea.
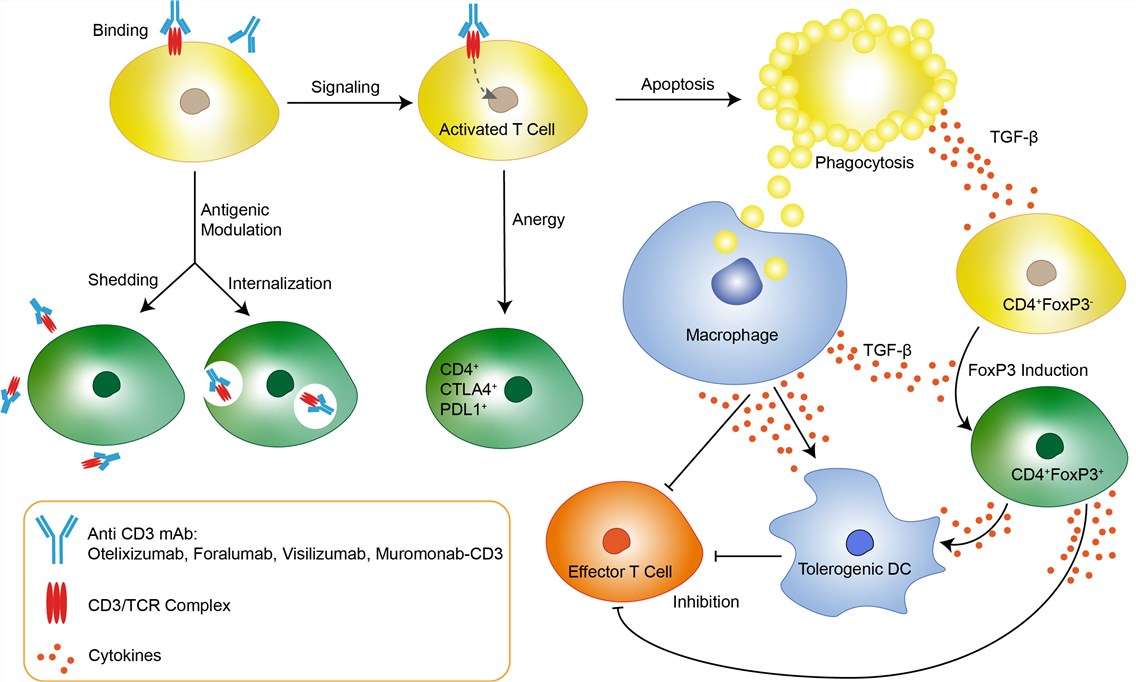 Fig 1. Mechanism of Action of Muromonab-CD3
Fig 1. Mechanism of Action of Muromonab-CD3
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

