Pembrolizumab Overview
Introduction of Pembrolizumab
Pembrolizumab (formerly MK-3475 and lambrolizumab) is a humanized monoclonal antibody (mAb) used in cancer immunotherapy. It is an IgG4 isotype antibody that blocks a protective mechanism of cancer cells, and allows the immune system to destroy those cancer cells. It targets the programmed cell death 1 (PD-1) receptor of lymphocytes. On September 4, 2014, the US Food and Drug Administration (FDA) approved pembrolizumab under the FDA Fast Track Development Program. It is approved for use following treatment with ipilimumab, or after treatment with Ipilimumab and a BRAF inhibitor in advanced melanoma patients who carry a BRAF mutation. In July 2015, pembrolizumab received marketing approval in Europe. On October 2, 2015, the FDA approved pembrolizumab for the treatment of metastatic non-small cell lung cancer (NSCLC) in patients whose tumors express PD-L1 and who have failed treatment with other chemotherapeutic agents. In May 2017, pembrolizumab received an accelerated approval from the FDA for use in any unresectable or metastatic solid tumor with DNA mismatch repair deficiencies or a microsatellite instability-high state (or, in the case of colon cancer, tumors that have progressed following chemotherapy). This approval marked the first instance in which the FDA approved marketing of a drug based only on the presence of a genetic mutation, with no limitation on the site of the cancer or the kind of tissue in which it originated. In June 2018, the FDA approved pembrolizumab for use in both advanced cervical cancer for PD-L1 positive patients and for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL), or who have relapsed after two or more prior lines of therapy.
Mechanism of Action of Pembrolizumab
PD-1 is the transmembrane programmed cell death 1 protein (also called PDCD1 and CD279). As an immune checkpoint molecular, it interacts with PD-L1 (PD-1 ligand 1, or CD274). PD-L1 on the cell surface binds to PD1 on an immune cell surface, which inhibits immune cell activity. Among PD-L1 functions is a key regulatory role on T cell activities. The PD-1 checkpoints function as a control over immune response hyperactivity. However, these immune checkpoints are also means by which tumors can inhibit T cells and block antitumor immune response. Interaction between PD-1 and PD-L1 and PD-L2 normally inhibits immune response by reducing T lymphocyte function. Signaling inhibits T cell activation, proliferation, and cytokine production. Regulation of T cell activation involves two complementary signals: TCR recognition of peptide/MHC and costimulatory signal provided by CD28 ligation to B7.1 or B7.2 on APCs. Many tumors express PD-L1 in order to induce negative regulation of T cells by the PD-1 checkpoint. Pembrolizumab inhibits checkpoint binding of PD-1 and PD-L1 between T cells and the tumor cells, thus inducing immune response.
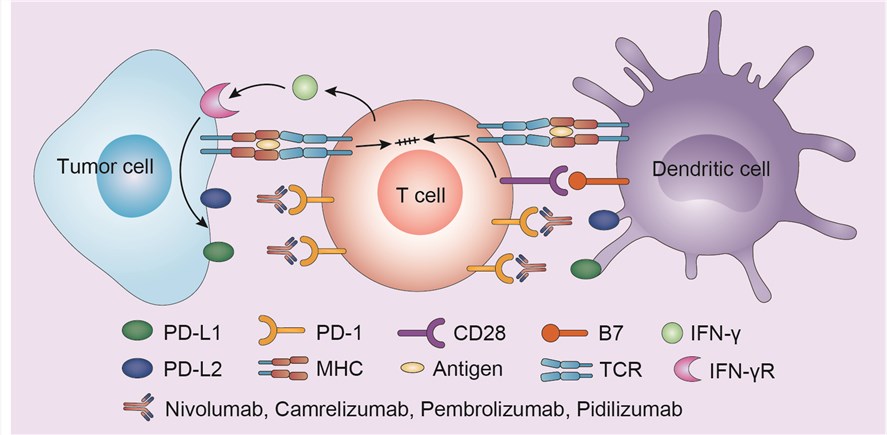 Fig.1 Mechanism of action of pembrolizumab
Fig.1 Mechanism of action of pembrolizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

