Pertuzumab Overview
Introduction of Pertuzumab
Pertuzumab (also called 2C4) is a humanized (from mouse) monoclonal antibody (mAb) used in combination with trastuzumab and docetaxel for the treatment of metastatic human epidermal growth factor receptor 2 (HER2)-positive breast cancer; it also used in the same combination as a neoadjuvant in early HER2-positive breast cancer. In 2012 the results were published of the CLEOPATRA trial, a randomized placebo-controlled Phase III trial of pertuzumab in combination with trastuzumab and docetaxel in HER2-positive metastatic breast cancer. Pertuzumab received US Food and Drug Administration (FDA) approval for the treatment of HER2-positive metastatic breast cancer later that year. The FDA approved the neoadjuvant indication in 2013. Pertuzumab was approved in Europe in 2013.
Mechanism of Action of Pertuzumab
The human epidermal growth factor receptor (HER) family comprises four type I transmembrane tyrosine kinase receptors (EGRFR or HER1, HER2, HER3 and HER4) with key roles in cell growth, proliferation, survival and oncogenesis. Each receptor consists of an extracellular segment with four domains involved in ligand binding and receptor dimerization, a transmembrane region, and an intracellular domain with kinase activity. Normally a bound ligand is necessary for receptor dimerization, except for the HER2 receptor that is constitutively available for dimerization and forms heterodimers with other ligand-activated HER receptors or homodimers in HER2- overexpressing cells. Pertuzumab is a humanized monoclonal antibody that binds the extracellular domain II of HER2. As a result, pertuzumab inhibits the ligand-mediated dimerization of HER2 by steric hindrance, inactivating multiple downstream signaling networks including the mitogen-activated protein kinase cascade (RAS/RAF/MEK/ERK) and the phosphoinositide 3-kinase (PI3K/AKT/mTOR) pathway. Complementary, trastuzumab acts mainly by inhibiting the ligand-independent HER2 signaling, preventing HER2 constitutive activation by extracellular domain cleavage. As it binds to the extracellular domain of HER2, pertuzumab can also induce an antibody-mediated immune effector function as seen with trastuzumab, but does not block HER2 shedding, furthermore, there is a growing evidence on the role of pertuzumab in increasing the trastuzumab-induced antibody-dependent cellular cytotoxicity (ADCC). The ADCC is a cell-mediated immune response triggered by antibodies that engage an immune system effector cell (typically natural killer cells) to lyse a target cell and releases cytokines such as interferon γ (IFNγ).
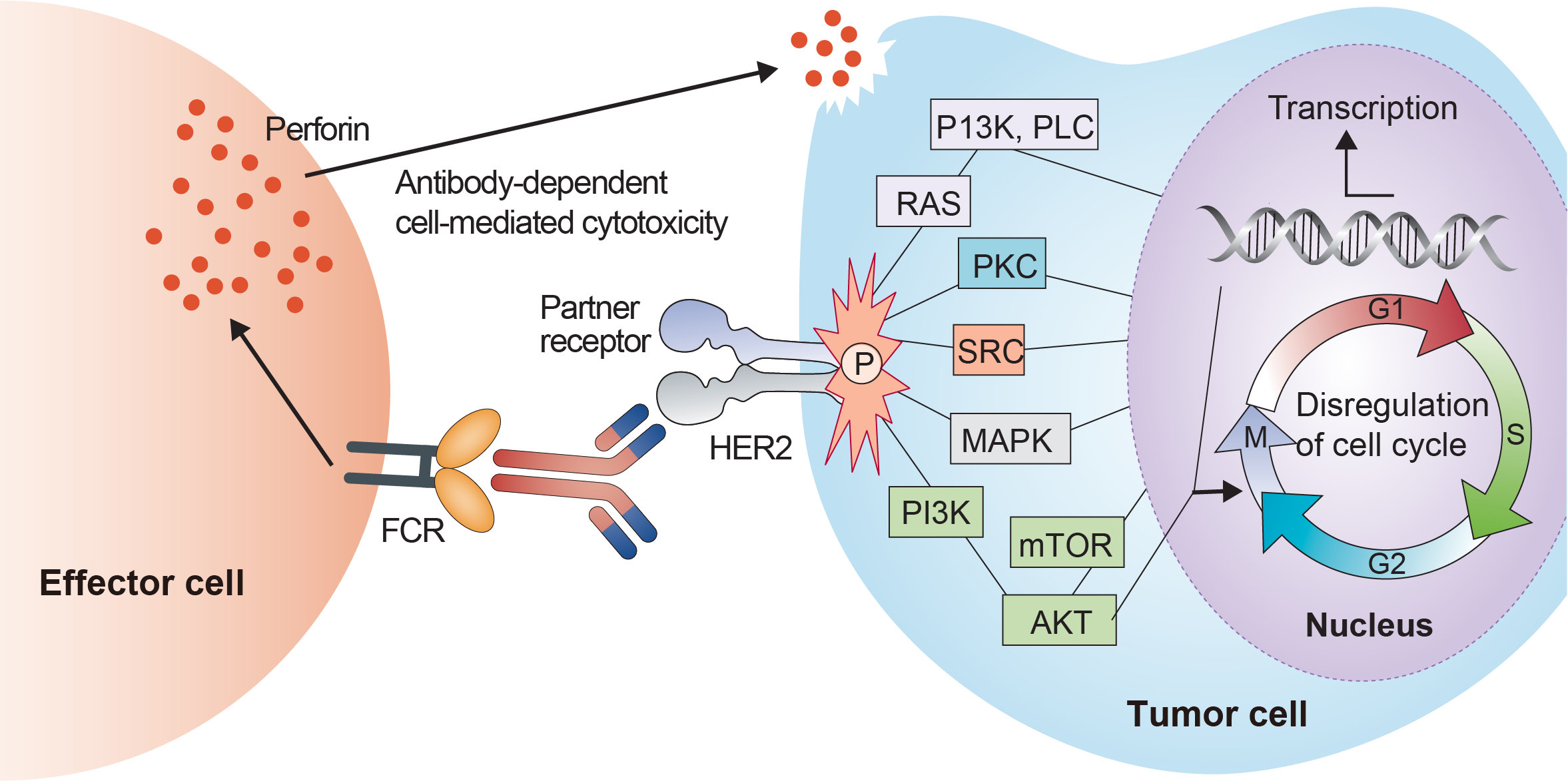 Fig.1 Mechanism of action of Pertuzumab
Fig.1 Mechanism of action of Pertuzumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



