pH/Enzyme Responsive Targeting-based Antibody Production Services
Are you currently facing challenges in achieving precise drug delivery, minimizing off-target toxicity, or enhancing therapeutic efficacy in complex biological environments? Our pH/enzyme responsive targeting-based antibody production services help you overcome these hurdles and unlock new therapeutic potential through advanced linker chemistry and innovative protein engineering techniques.
Introduction
The pH/enzyme responsive targeting-based antibody production services represent a cutting-edge approach in precision medicine, enabling the development of therapeutics that selectively release their payload at diseased sites. This strategy capitalizes on the unique biochemical characteristics of pathological microenvironments, such as the lower pH in tumors or the overexpression of specific enzymes. By designing smart linkers that remain stable in systemic circulation but rapidly cleave under these specific conditions, we can significantly enhance therapeutic efficacy and reduce systemic toxicity.
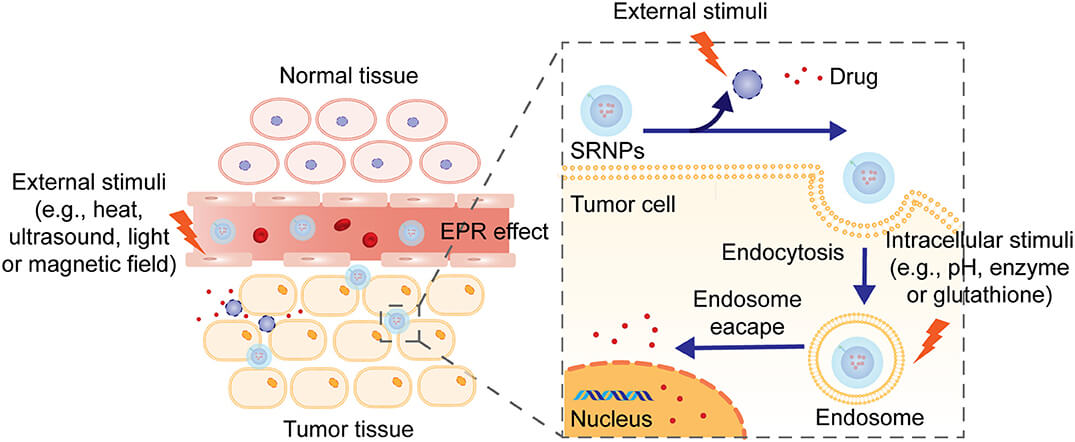 Fig.1 Stimulus-responsive drug release mechanisms.1,3
Fig.1 Stimulus-responsive drug release mechanisms.1,3
pH/Enzyme Responsive Targeting-based Antibody Production Services at Creative Biolabs
At Creative Biolabs, we specialize in developing sophisticated antibody-drug conjugates (ADCs) and other bioconjugates that leverage the unique pH and enzymatic differences in diseased tissues, particularly in the tumor microenvironment. These services focus on developing "smart" antibody constructs that release their therapeutic payload only under specific conditions prevalent in the diseased tissue, maximizing safety and efficacy.
Our services deliver precisely engineered therapeutic agents designed for optimal stability in circulation and controlled, localized release at the target site, significantly enhancing therapeutic efficacy while minimizing systemic side effects.
How It Works
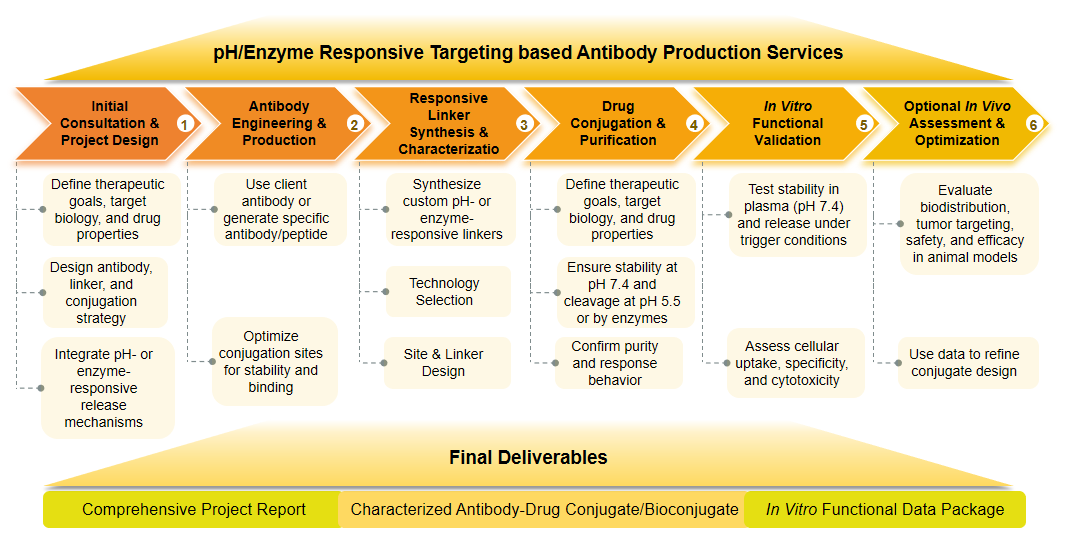
Types of our pH/Enzyme Responsive Targeting-based Antibody Production Services
Smart Release Antibody Production
This service focuses on the design, synthesis, and conjugation of antibodies or targeting moieties with drug payloads via pH- or enzyme-responsive linkers. The goal is to create conjugates that exhibit optimal stability in systemic circulation and trigger precise, controlled drug release only upon encountering specific pH levels or enzymatic activity within the target tissue. This includes the development of traceless linkers and the integration of advanced conjugation chemistries for high-quality ADCs and protein-drug conjugates.
Controlled Release Kinetic Evaluation
Beyond production, we provide comprehensive analytical services to characterize the release kinetics of your responsive conjugates. Utilizing advanced analytical techniques (e.g., HPLC, mass spectrometry), we precisely measure drug release rates under various simulated physiological and pathological conditions (e.g., different pH values, presence of specific enzymes, plasma). This data is crucial for understanding the in vitro performance of your conjugate and predicting it in vivo behavior, enabling informed optimization.
Benefits for You
- One-stop Integrated Solution: We provide comprehensive services from responsive linker design and antibody engineering to drug conjugation and rigorous in vitro functional validation.
- Efficient Process Development: Our streamlined upstream and downstream processes ensure efficient synthesis and purification, maximizing yields and minimizing turnaround times.
- Customized Linker Design: Leveraging deep chemical biology expertise, we design and synthesize bespoke pH-responsive and enzyme-responsive linkers tailored to your payload and target.
- Robust Quality Assurance: Our services adhere to a well-established quality system, incorporating robust quality assurance and process analytical technology for guaranteed product purity, stability, and activity.
- Optimized Conjugation & Release: We meticulously optimize conjugation for desired DAR and in vivo stability, precisely tuning release kinetics for maximum targeted payload delivery.
- Comprehensive Functional Validation: We conduct in-depth in vitro assays, including stability, triggered release, cellular uptake, and cytotoxicity, providing a complete performance profile.
- Expert Consultation: Our experienced biology experts offer continuous scientific consultation, guiding you through every project stage to meet your specific needs.
Case Study
Summary: This study investigates L19-IL2, an antibody-cytokine fusion protein that targets a specific fibronectin variant in pancreatic ductal adenocarcinoma. Using syngeneic mouse models and 3D tumor spheroids, it shows that L19-IL2 has dose-dependent anti-tumor effects, boosting the infiltration of T-lymphocytes and their ability to kill cancer cells by increasing substances like granzymes, perforins, and IL-2 receptors. When combined with a chemotherapy regimen, it reduces tumor growth and extends survival more effectively than either treatment alone.
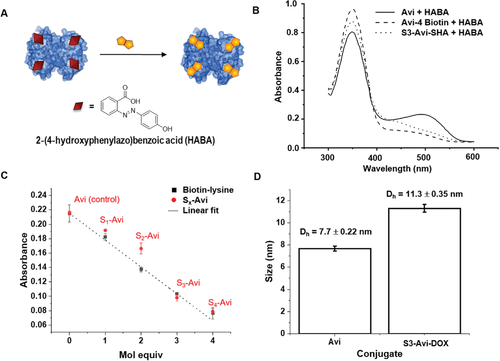 Fig.2 Competitive binding assay technique to analyze biomolecular interactions.2,3
Fig.2 Competitive binding assay technique to analyze biomolecular interactions.2,3
FAQs
How do your pH/enzyme responsive linkers ensure drug specificity and minimize off-target effects?
Our linkers are meticulously designed to remain highly stable at physiological pH (around 7.4) and in the bloodstream. They are engineered to specifically respond to the unique conditions found in the target microenvironment, such as the lower pH (e.g., 5.5-6.5) in tumors or the elevated activity of certain enzymes. This precise triggering mechanism ensures that the therapeutic payload is released predominantly at the disease site, significantly reducing systemic exposure and potential off-target toxicity.
What types of drug payloads can be conjugated using your responsive linker technology?
Our versatile linker platforms are designed to accommodate a broad range of therapeutic payloads. We have successfully conjugated various molecules, including small molecule cytotoxic drugs, photosensitizers, and therapeutic peptides (e.g., PD-L1 antagonists). The chemistry allows for binding to primary amines, alcohols, and phenols, providing significant flexibility for diverse drug candidates.
How do your responsive linker technologies compare to conventional, non-cleavable linkers in ADCs?
Our responsive linker technologies offer a significant advantage over conventional non-cleavable linkers by enabling controlled drug release. While non-cleavable linkers rely on antibody degradation within lysosomes for drug release, our pH/enzyme-responsive linkers provide an additional layer of control, ensuring active drug release specifically in response to the unique pathological microenvironment.
Why Choose Us?
Creative Biolabs stands at the forefront of pH/enzyme responsive targeting-based antibody production services, offering unparalleled expertise and innovative platforms that translate into tangible benefits for your projects. Our commitment to scientific rigor, quality, and client success ensures that your therapeutic development is in expert hands.
Customer Reviews:
We approached Creative Biolabs with a complex antibody-drug conjugate project requiring a traceless, pH-sensitive linker. Their team not only synthesized a highly effective gallic acid-derived linker but also provided comprehensive characterization data that simplified our downstream analysis. The non-toxic nature of their linker platform was a significant advantage over alternatives we had considered, reducing potential safety concerns. Their integrated approach from design to in vitro validation saved us considerable time and resources. — 1 Year Ago, Dr. An H*k
Using Creative Biolabs' pH/enzyme responsive targeting services in our research has significantly improved the precision of our drug delivery. The ability to design linkers that specifically respond to the acidic tumor microenvironment has been a game-changer, allowing us to see far less off-target effects compared to our previous non-responsive constructs. The stability in plasma was also exceptional, which is a crucial consideration for in vivo applications. - 5 Months Ago, Dr. La M*n
How to Contact Us
Ready to revolutionize your drug delivery strategy? Contact our team of experts today to discuss your specific project requirements and discover how our pH/enzyme responsive targeting-based antibody production services can accelerate your therapeutic development.
References
- Li, Mengqian et al. "Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery." Frontiers in chemistry vol. 8 647. 30 Jul. 2020. DOI: https://doi.org/10.3389/fchem.2020.00647
- Raabe, Marco et al. "Assembly of pH-Responsive Antibody-Drug-Inspired Conjugates." Macromolecular bioscience vol. 22,2 (2022): e2100299. DOI: https://doi.org/10.1002/mabi.202100299
- Distributed under Open Access License CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

