Protein Purification Protocol & Troubleshooting
Protein purification is a process aimed at separating one or several proteins from a complex mixture. This technique is widely used in biochemical research applications. Purification of the protein of interest is fundamental to the study of protein function, structure, and interactions. We offer a basic protocol for protein purification that focuses on four basic steps, including cell lysis, protein binding to the matrix, washing, and elution. Our goal is to keep as much functional protein as possible while removing as many contaminants as possible.
Solutions and Reagents
| Stages | Solutions and Reagents |
| Sample Preparation | Cell lysis buffer and washing buffer |
| Purification | Affinity chromatography matrices, ligands, equilibration buffer, washing buffer, elution buffer |
Protein Purification Procedure
There are several common methods of protein purification, including salinization, dialysis, ion exchange, gel filtration, and affinity chromatography. Affinity chromatography is one of the most reliable and commonly used methods. This takes advantage of the specific and reversible binding of proteins to matrix-binding ligands.
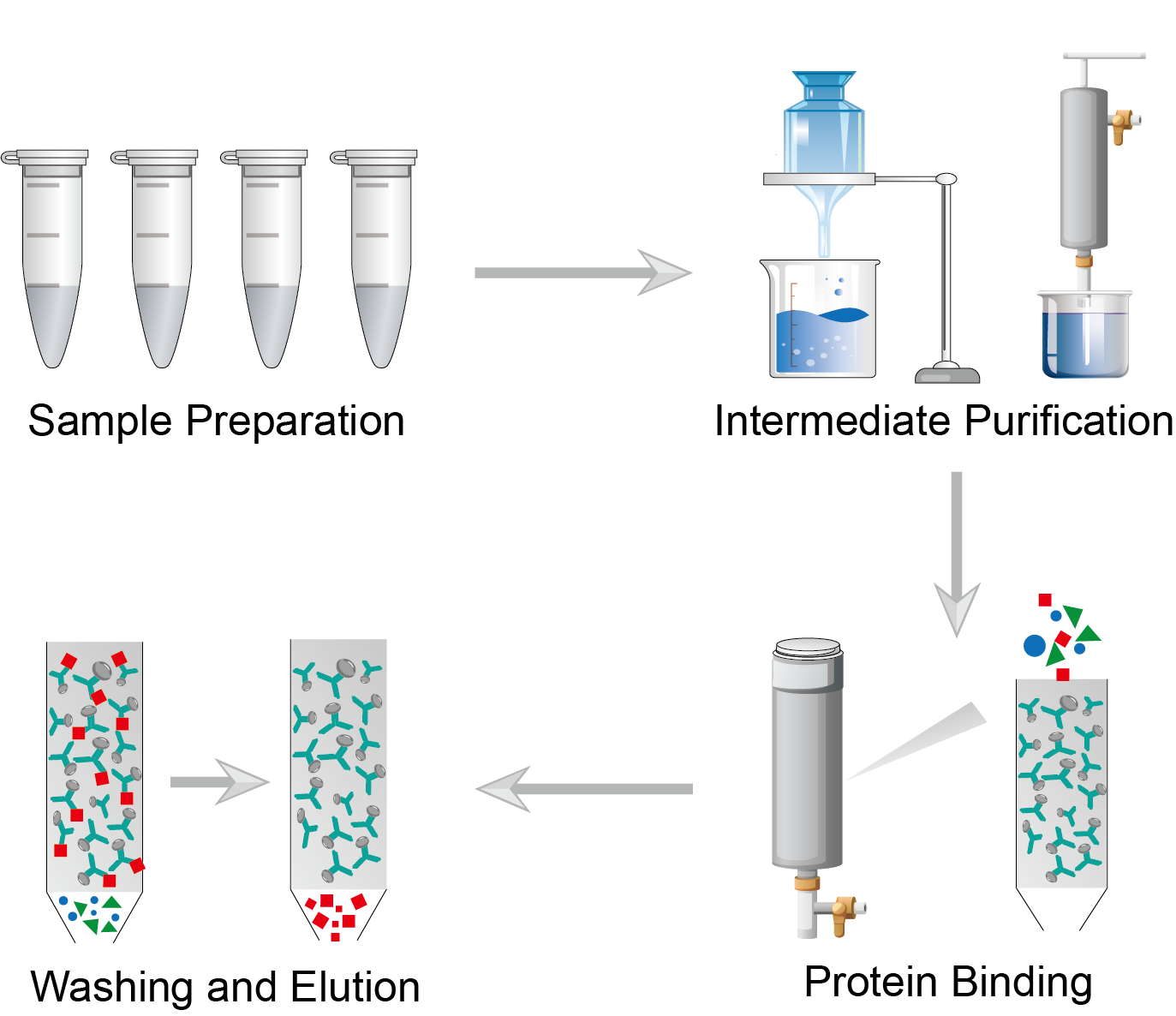 Figure 1: Live Cell Imaging Procedure
Figure 1: Live Cell Imaging Procedure
Perform crude protein sample preparation, including cell harvesting, cell crushing, and clarification. First, isolate and harvest cells from the culture medium. Then perform cell fragmentation, usually using enzymatic, detergent, and sonication methods. Finally, the separation of proteins is achieved by filtration or centrifugation.
Next, the proteins in the crude extract can be initially purified by removing impurity proteins through salinization, dialysis, and gel filtration steps. You can choose a purification protocol with different principles depending on your sample.
Select porous carrier materials such as agarose, polyacrylamide, cellulose, and silica to make affinity chromatography matrices. Select affinity chromatography ligands according to the protein molecules to be separated. The ligands are covalently immobilized or adsorbed on the surface of the affinity chromatography matrix by biological interactions. Fill the column with the matrix to form the stationary phase. Apply the sample to the column. As the protein mixture passes through the chromatographic column, proteins with immobilized substrate binding sites bind to the immobilization.
Wash the column with wash buffer immediately after the sample enters the column. Impurity protein molecules that have not been bound are washed away. Then elute the non-specifically bound proteins with an elution buffer. Then, use another elution buffer to elute the specifically bound proteins. After all the specifically bound proteins are eluted from the column, wash the column with eluent.
Troubleshooting
We are committed to getting your experiment back on track. Check out our troubleshooting guides for common issue scenarios.
No protein in the eluate
- Sample causes. One possibility is that the target protein level is too low and below the binding threshold. If you are using an affinity column, try using a higher input amount. Another possibility is that the protein is not expressed. If it is a recombinant protein expressed in an induction system, check that the induction is working properly. You can choose from commercially available antibodies against a variety of tags to determine the level of construct expression.
- Protein aggregation causes. Protein aggregation in the column results in non-elution. We recommend that you adjust the buffer conditions to obtain higher protein stability.
- Elution solution causes. The elution solution is not properly prepared and the protein remains bound to the column. You should prepare a fresh elution solution and repeat the elution.
High background
- Washing causes. Washing is not stringent enough. You can take the following steps, including additional wash steps or optimizing your wash buffer composition.
- Buffer causes. The wrong buffer causes a high background. Consider additional purification steps or reduce the total amount of protein sampled on the column.
- Resin causes. The resin binds to specific proteins, causing contamination with impurities. You may consider additional purification steps or reducing the total amount of protein sampled on the column.
Low resolution
- Flow rate causes. Faster or slower than optimal flow rates can cause this. You can change the flow rate until the optimal value is reached.
- Column causes. Inadequate cleaning of the chromatographic column and protein aggregation on the column. We recommend using a more stringent buffer, or adjusting the characteristics of the buffer (pH, concentration).
- Elution causes. Elution conditions lack rigor. You can improve by adjusting the characteristics of the buffer or increasing the concentration of competitors.
Protein does not bind
- Insufficient binding time causes. Inability to bind due to insufficient time. Please reduce the flow rate or stop the column to allow for incubation.
- Buffer causes. Binding conditions are not appropriate. The properties of the buffer (pH, concentration) can be adjusted to achieve optimal binding conditions.
- Protein tag causes. The tag is not translated or cannot be bound. Check the plasmid sequence or move the tag to a different position to ensure that binding proceeds.
Protein inactivation
- Sample causes. Proteins are degraded in the starting material due to high temperatures or repeated freezing and thawing. Your samples should be ground in liquid nitrogen before being processed on ice or in a cold room at 4°C. For storage, freeze quickly and store your samples at -80°C.
- Buffer causes. Adjust buffer conditions for higher protein stability.
- Purification causes. The cofactors required for activity are removed during the purification process. You can add appropriate cofactors to ensure protein activity.
We are committed to the success of your protein purification. We hope the information above will put you on the right path in your protein research. If you still have any questions, please contact our technical support staff for additional online resources.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



