Rituximab Biosimilar Pipeline Development Service
Comprehensive Solutions for Rituximab Biosimilar Development
At Creative Biolabs, we offer comprehensive Rituximab biosimilar development services, leveraging our expertise in recombinant antibody production and biosimilar manufacturing. Our services cover the entire biosimilar pipeline, from early-stage discovery and characterization to large-scale production, providing you with the tools and support necessary to develop a high-quality, regulatory-compliant Rituximab biosimilar.
Our development process begins with in-depth analysis of the reference Rituximab, ensuring accurate replication of its structural, functional, and glycosylation profiles. We then proceed with optimizing expression systems and developing stable cell lines capable of producing large quantities of the biosimilar. Analytical characterization, including potency, purity, and identity testing, is conducted at each stage to ensure that the biosimilar is comparable to the reference product.
By combining our extensive experience, state-of-the-art technology, and regulatory expertise, Creative Biolabs ensures that your Rituximab biosimilar development is efficient, scientifically rigorous, and in full compliance with global regulatory standards, enabling you to bring a high-quality product to market with confidence.
- At a glance of Rituximab
Basic Information
Drug Type: Monoclonal antibody
Synonyms: IDEC-C2B8-anti-CD20; Rituximab (Genetical Recombination)
Target: CD20
Action: Inhibitors
Mechanism: CD20 inhibitors(B-lymphocyte antigen CD20 inhibitors); ADCC(Antibody-dependent cell-mediated cytotoxicity (ADCC) effects); CD20-directed cytolytic effects
Therapeutic Areas: Neoplasms; Immune System Diseases; Infectious Diseases
Active Indication: Cardiac transplant rejection; Liver transplant rejection; Lung transplant rejection
Inactive Indication: Acute bilineal leukemia; Acute Graft Versus Host Disease; Adult Acute Myeloblastic Leukemia
First Approval Date: United States (26 Nov 1997), Non-Hodgkin Lymphoma
Most Recent Events
| Date | Events |
| 2025-05-10 | Rituximab biosimilars show improved efficacy in patients with rheumatoid arthritis in recent phase III trials. |
| 2025-02-15 | The FDA approves a new subcutaneous formulation of Rituximab for the treatment of non-Hodgkin lymphoma (NHL). |
| 2024-11-20 | Rituximab demonstrates effectiveness in new indication for autoimmune hemolytic anemia (AIHA) in an updated clinical study. |
| 2024-08-12 | A head-to-head comparison of Rituximab and a novel monoclonal antibody shows superior outcomes in treating B-cell malignancies. |
| 2024-04-03 | Rituximab biosimilars gain approval in the EU for treatment of chronic lymphocytic leukemia (CLL), expanding market access. |
- Pipeline Status
Our Rituximab Biosimilar Pipeline Offers
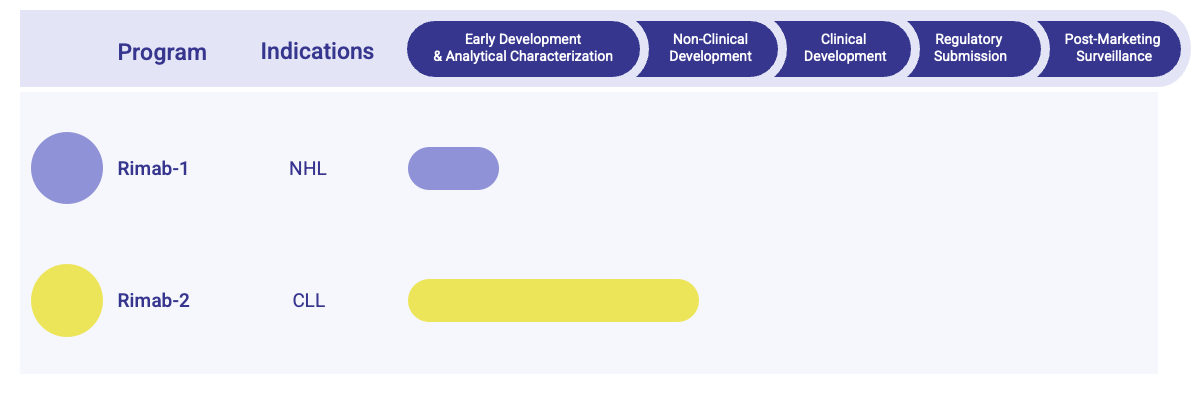
Development Workflow of Rituximab Biosimilar Pipeline
| Early Development & Analytical Characterization | ||
| 1 | Sequence and Structural Characterization | Detailed analysis of the reference Rituximab to confirm the amino acid sequence and overall structure, ensuring that all critical attributes, including disulfide bonding, are replicated accurately. |
| 2 | Cell Line Development | Creation of high-yield, stable mammalian cell lines (such as CHO or HEK293) for biosimilar production. We ensure that the cell lines are optimized to produce large amounts of the biosimilar. |
| 3 | Expression System Optimization | Selection of the most suitable expression platform (CHO, HEK293, etc.) for efficient, scalable production. |
| 4 | Glycosylation Profiling | Analysis of glycosylation patterns to ensure the biosimilar has identical or highly similar post-translational modifications compared to the reference product. |
| 5 | Early Analytical Testing | Using HPLC, ELISA, and mass spectrometry, we perform identity, purity, potency, and integrity tests to ensure that the biosimilar is structurally and functionally equivalent to the reference Rituximab. |
| Non-Clinical Development | ||
| 6 | In Vitro Biological Activity Testing | Functional assays to test the biosimilar's ability to engage target antigens, induce ADCC (antibody-dependent cellular cytotoxicity), and participate in CDC (complement-dependent cytotoxicity). |
| 7 | In Vivo Pharmacology & Toxicology | Comprehensive animal studies to assess the pharmacokinetics (PK), pharmacodynamics (PD), and safety profile of the biosimilar. |
| 8 | Immunogenicity Testing | In vitro and in vivo studies to assess the likelihood of anti-drug antibody (ADA) formation and immune responses against the biosimilar. |
| 9 | Biodistribution Studies | These studies assess the distribution of the biosimilar in the body and help determine potential accumulation in specific tissues. |
- Available Packages
At Creative Biolabs, we offer a range of service packages tailored to support every stage of your Rituximab biosimilar development journey. Each package is designed to provide a comprehensive and efficient approach, ensuring that you meet your project goals while adhering to the highest industry standards. Below are the available packages:
| NO. | Item Name | Includes | Deliverables |
| 1 | Early Development & Analytical Characterization Package |
|
|
| 2 | Non-Clinical Development Package |
|
|
| 3 | Full Biosimilar Development Package |
|
|
- Why Choose Us
At Creative Biolabs, we are dedicated to providing industry-leading services for the development of Rituximab biosimilars. With years of experience and a proven track record in recombinant antibody technology, we offer unparalleled expertise and a comprehensive range of solutions. Here’s why you should choose us for your biosimilar development:
Expertise in Biosimilar Development
Our team has extensive experience in the development of biosimilars, ensuring scientific excellence, quality, and efficiency at every stage of your Rituximab biosimilar project.
End-to-End Services
We provide comprehensive services from early-stage characterization and cell line development to non-clinical testing. Our integrated approach streamlines the entire process.
Customized Solutions
We understand that every project is unique. Our services are fully customizable, tailored to your specific needs, timelines, and budget, ensuring optimal outcomes.
Partnering with Creative Biolabs means gaining a reliable, experienced team that is committed to helping you bring your Rituximab biosimilar to market with confidence and precision.
- Ready to Advance Your Rituximab Biosimilar Program
Ready to start your Rituximab biosimilar development or have any questions about our services? Our team is here to assist you at every step of the way.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



