 Loading...
Loading...

SRC
Cancer-related genes, Disease related genes, Enzymes, FDA approved drug targets, Human disease related genes, Metabolic proteins, Plasma proteins, Transporters
Intracellular
Cell type enhanced (Langerhans cells, Gastric mucus-secreting cells, Distal enterocytes)
Immune cell enhanced (intermediate monocyte, non-classical monocyte)
Cell line enhanced (A549, HBEC3-KT, RT4)
Part of a complex comprised of PTPRA, BCAR1, BCAR3 (via SH2 domain) and SRC; the formation of the complex is dependent on integrin mediated-tyrosine phosphorylation of PTPRA (PubMed:22801373). Interacts with DDEF1/ASAP1; via the SH3 domain (By similarity). Interacts with CCPG1 (By similarity). Identified in a complex containing FGFR4, NCAM1, CDH2, PLCG1, FRS2, SRC, SHC1, GAP43 and CTTN (By similarity). Interacts with ERBB2, STAT1 and PNN (By similarity). Interacts with DDR1, DDR2 and DAB2 (By similarity). Interacts with CDCP1, TGFB1I1 and TOM1L2 (PubMed:15851033, PubMed:16479011, PubMed:17202804). Interacts with the cytoplasmic domain of MUC1, phosphorylates it and increases binding of MUC1 with beta-catenin (PubMed:11152665). Interacts with RALGPS1; via the SH3 domain (PubMed:10747847). Interacts with CAV2 (tyrosine phosphorylated form) (PubMed:12091389, PubMed:15504032). Interacts (via the SH3 domain and the protein kinase domain) with ARRB1; the interaction is independent of the phosphorylation state of SRC C-terminus (By similarity). Interacts with ARRB1 and ARRB2 (PubMed:10753943, PubMed:9924018). Interacts with SRCIN1 (PubMed:17525734). Interacts with NDFIP2 and more weakly with NDFIP1 (PubMed:20534535). Interacts with PIK3CA and/or PIK3C2B, PTK2/FAK1 and ESR1 (dimethylated on arginine) (PubMed:18657504, PubMed:21411625). Interacts with FASLG (PubMed:19807924). Interacts (via SH2 domain) with the 'Tyr-402' phosphorylated form of PTK2B/PYK2 (PubMed:14585963). Interacts (via SH2 domain) with FLT3 (tyrosine phosphorylated) (By similarity). Interacts with PDGFRA (tyrosine phosphorylated) (By similarity). Interacts with CSF1R (By similarity). Interacts (via SH2 and SH3 domain) with TNK2 (PubMed:21309750). Interacts (via protein kinase domain) with the tyrosine phosphorylated form of RUNX3 (via runt domain) (PubMed:20100835). Interacts with TRAF3 (via RING-type zinc finger domain) (PubMed:19419966). Interacts with DDX58, MAVS and TBK1 (PubMed:19419966). Interacts (via SH2 domain) with RACK1; the interaction is enhanced by tyrosine phosphorylation of RACK1 and inhibits SRC activity (PubMed:9584165, PubMed:11279199). Interacts with EPHB1; activates the MAPK/ERK cascade to regulate cell migration (PubMed:12925710). Interacts with FCAMR (PubMed:8759729). Interacts (via SH2 domain) with the 'Tyr-9' phosphorylated form of PDPK1 (PubMed:18024423). Interacts with AMOTL2; this interaction regulates the translocation of phosphorylated SRC to peripheral cell-matrix adhesion sites (PubMed:17293535). Interacts with TRAP1 (PubMed:23564345). Interacts with CBLC; the interaction is enhanced when SRC is phosphorylated at Tyr-419 (PubMed:14661060, PubMed:22888118). Interacts with ARHGEF5 (By similarity). Interacts (via cytoplasmic domain) with CEACAM1 (via SH2 domain); this interaction is regulated by trans-homophilic cell adhesion (PubMed:7478590). Interacts with MPP2 (PubMed:19665017). Interacts with PRR7 (PubMed:21460222). Interacts (via kinase domain and to a lesser extent the SH2 domain) directly with PDLIM4; this interaction results in PTPN13-mediated dephosphorylation of this protein leading to its inactivation (PubMed:19307596). Interacts with P85 (PIK3R1 or PIK3R2) (PubMed:28903391). Interacts with HNRNPA2B1 (PubMed:31320558). Interacts with IL6ST/gp130 (PubMed:25731159). Interacts (via SH3 domain) with PELP1 in the presence of 17-beta-estradiol. Interacts with AMBRA1 (By similarity). (Microbial infection) Interacts with HEV ORF3 protein; via the SH3 domain. (Microbial infection) Interacts (via SH2 domain) with HCV non-structural protein 5A (via N-terminus).
Kinase, Transferase, Tyrosine-protein kinase
- Recombinant Rabbit Anti-SRC Monoclonal Antibody (JF0947) (MRO-1431-CN)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC, FC
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC
-
- Species Reactivity: Human
- Type: IgG
- Application: WB, IF, ICC, FC
-
- Species Reactivity: Chicken
- Type: Mouse antibody
- Application: ICC, WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, FC
- Rabbit Anti-SRC Polyclonal Antibody (MRO-2199-CN) (MRO-2199-CN)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC, FC
-
- Derivation: Phage display library screening
- Species Reactivity: Human
- Type: IgG
- Application: IP, WB, Dot, ICC/IF
- Recombinant Rabbit Anti-SRC Antibody (clone R05-7H8) (VS3-FY1389)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Rat
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB
- Rabbit Anti-SRC Recombinant Antibody (VS3-CJ623) (VS3-CJ623)
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IHC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, FC
- Mouse Anti-SRC Recombinant Antibody (VS3-CJ621) (VS3-CJ621)
-
- Species Reactivity: Human
- Type: Mouse IgG2b, κ
- Application: WB
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: ELISA, WB, IF, FC
- Mouse Anti-SRC Recombinant Antibody (clone 5D10C4) (VS3-XY1463)
-
- Species Reactivity: Human
- Type: Mouse IgG2a
- Application: ELISA, WB
- Mouse Anti-SRC Recombinant Antibody (clone 4F1E8) (VS3-XY1462)
-
- Species Reactivity: Human
- Type: Mouse IgG2b
- Application: ELISA, WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Mouse IgG
- Application: WB, ELISA
- Mouse Anti-SRC Recombinant Antibody (clone 1F11) (VS3-QX1053)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, IHC, ICC, FC, ELISA
- Mouse Anti-SRC Recombinant Antibody (clone 17AT28) (VS3-QX1052)
-
- Derivation: Mouse
- Species Reactivity: Human, Mouse
- Type: Mouse IgG1
- Application: WB, ICC
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: WB, ELISA
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
For Research Use Only. Not For Clinical Use.

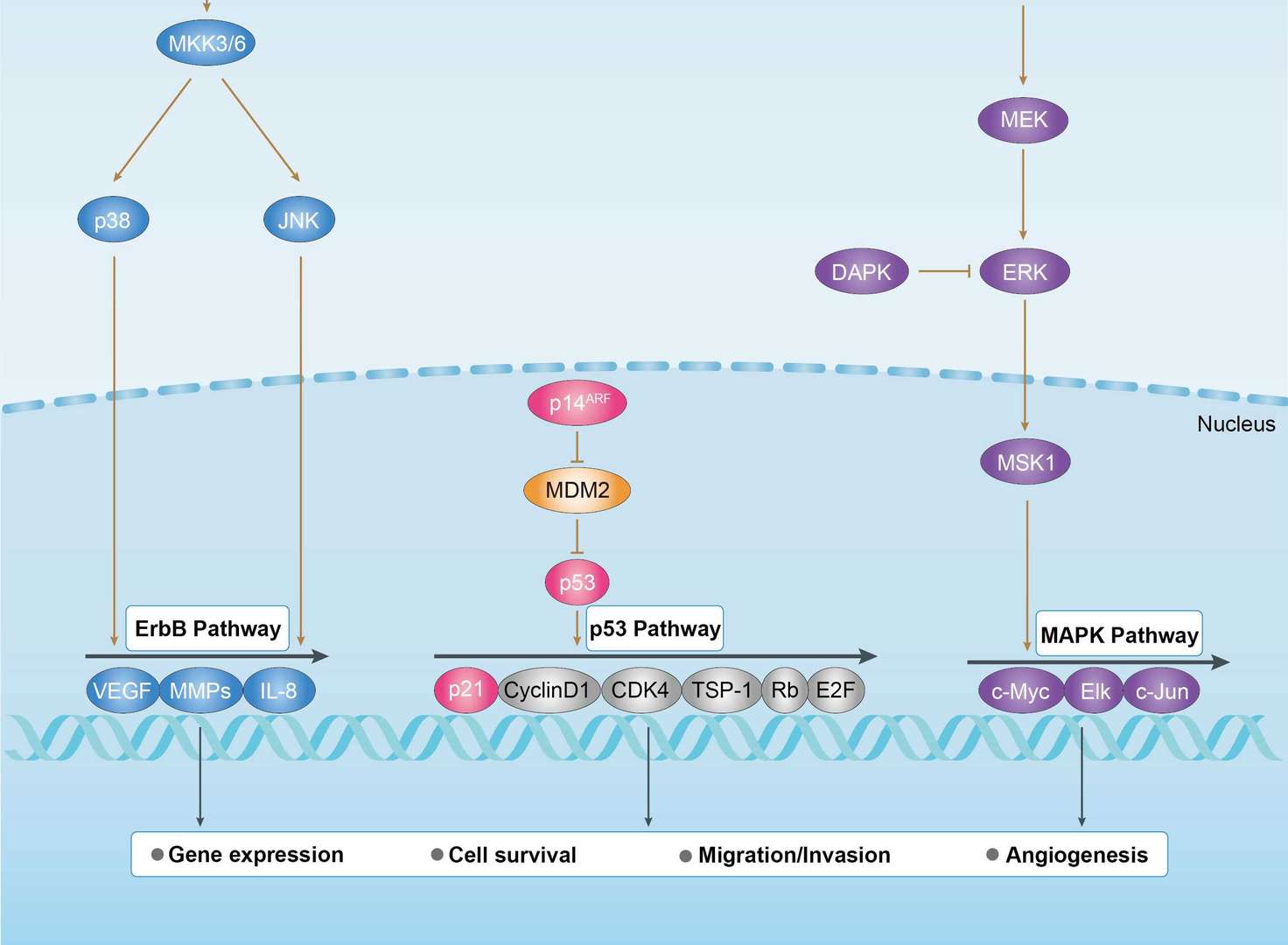 Bladder Cancer
Bladder Cancer
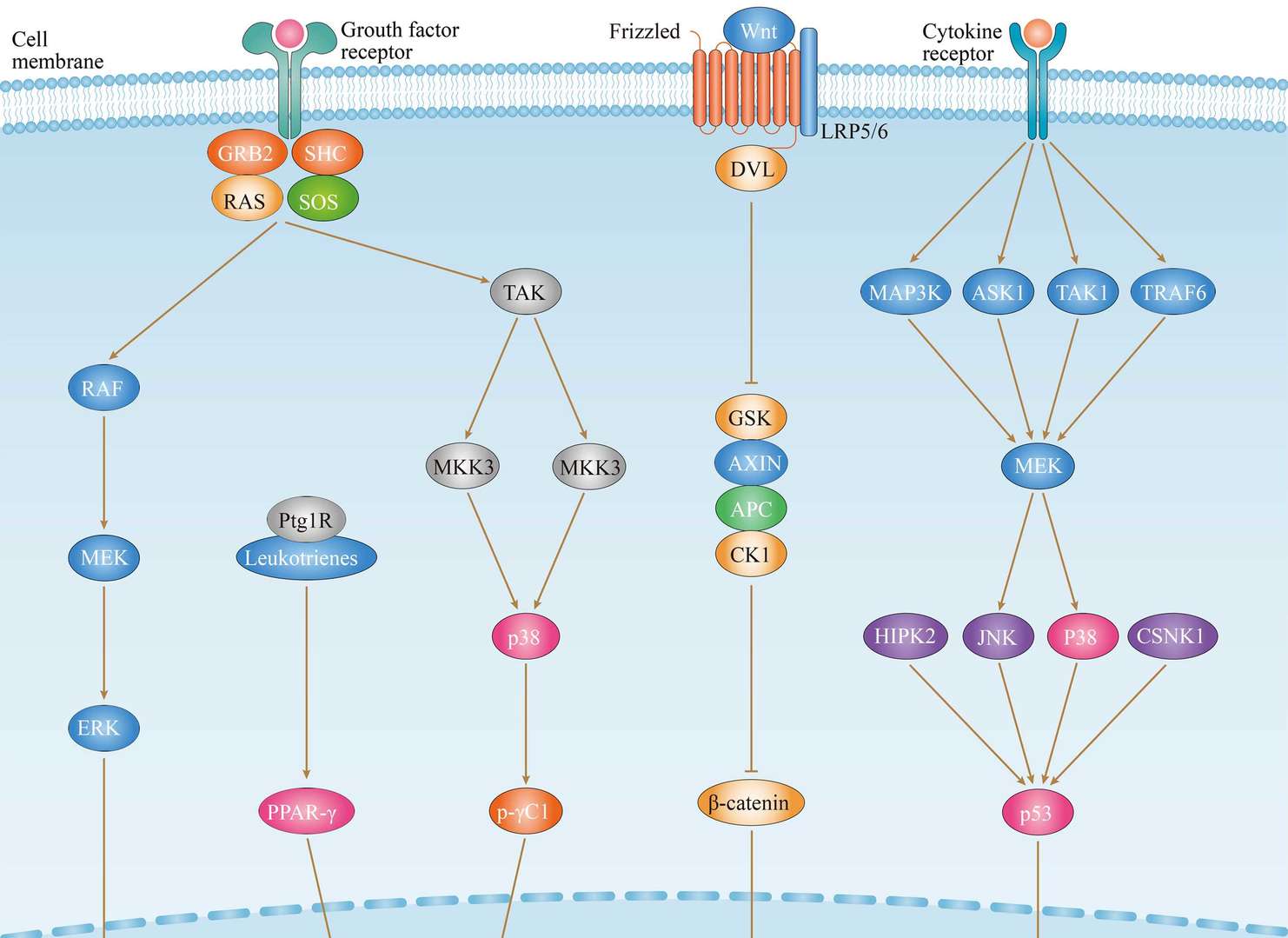 Throid Cancer
Throid Cancer
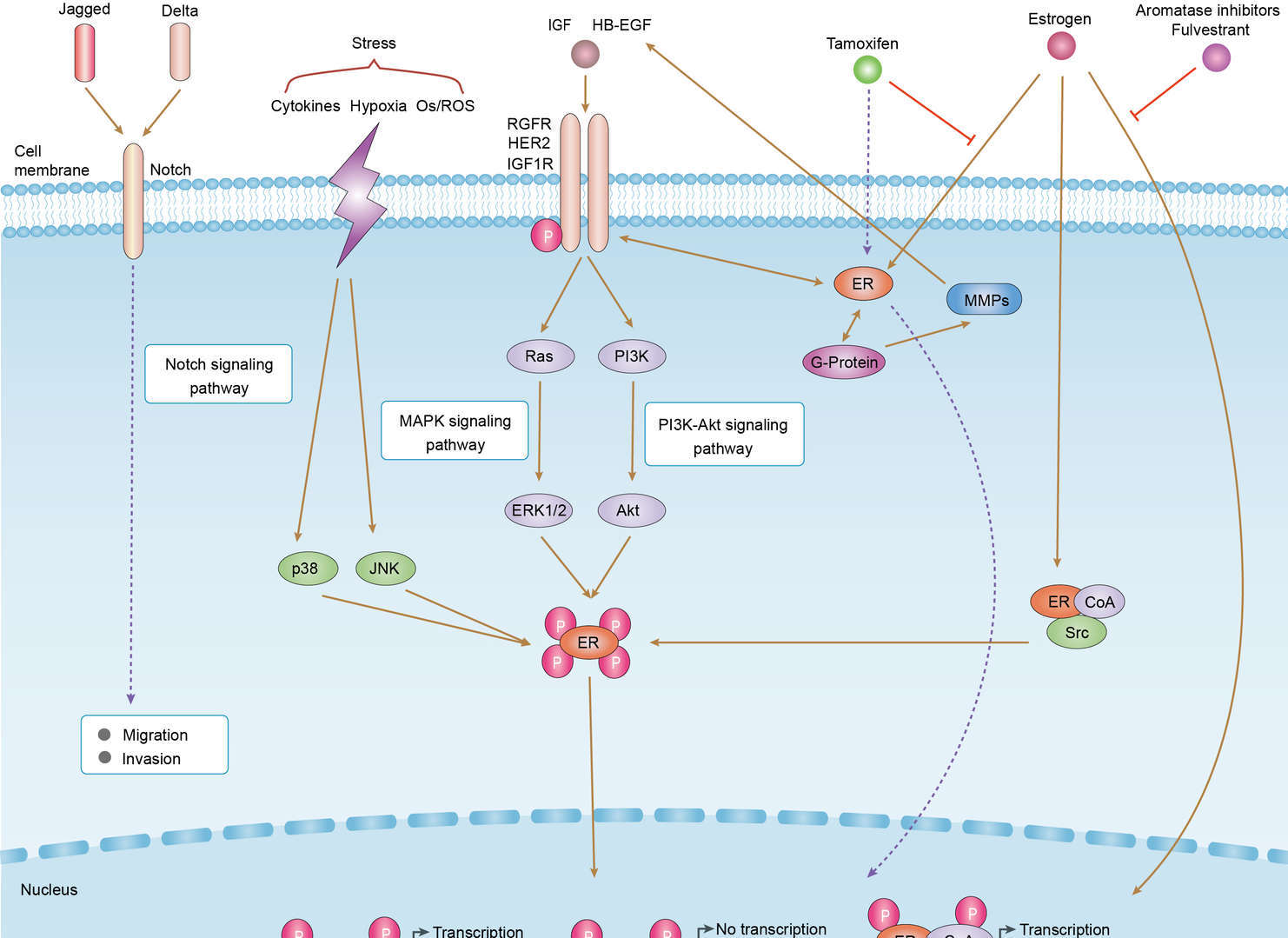 Endocrine Resistance
Endocrine Resistance
 cAMP Signaling Pathway
cAMP Signaling Pathway
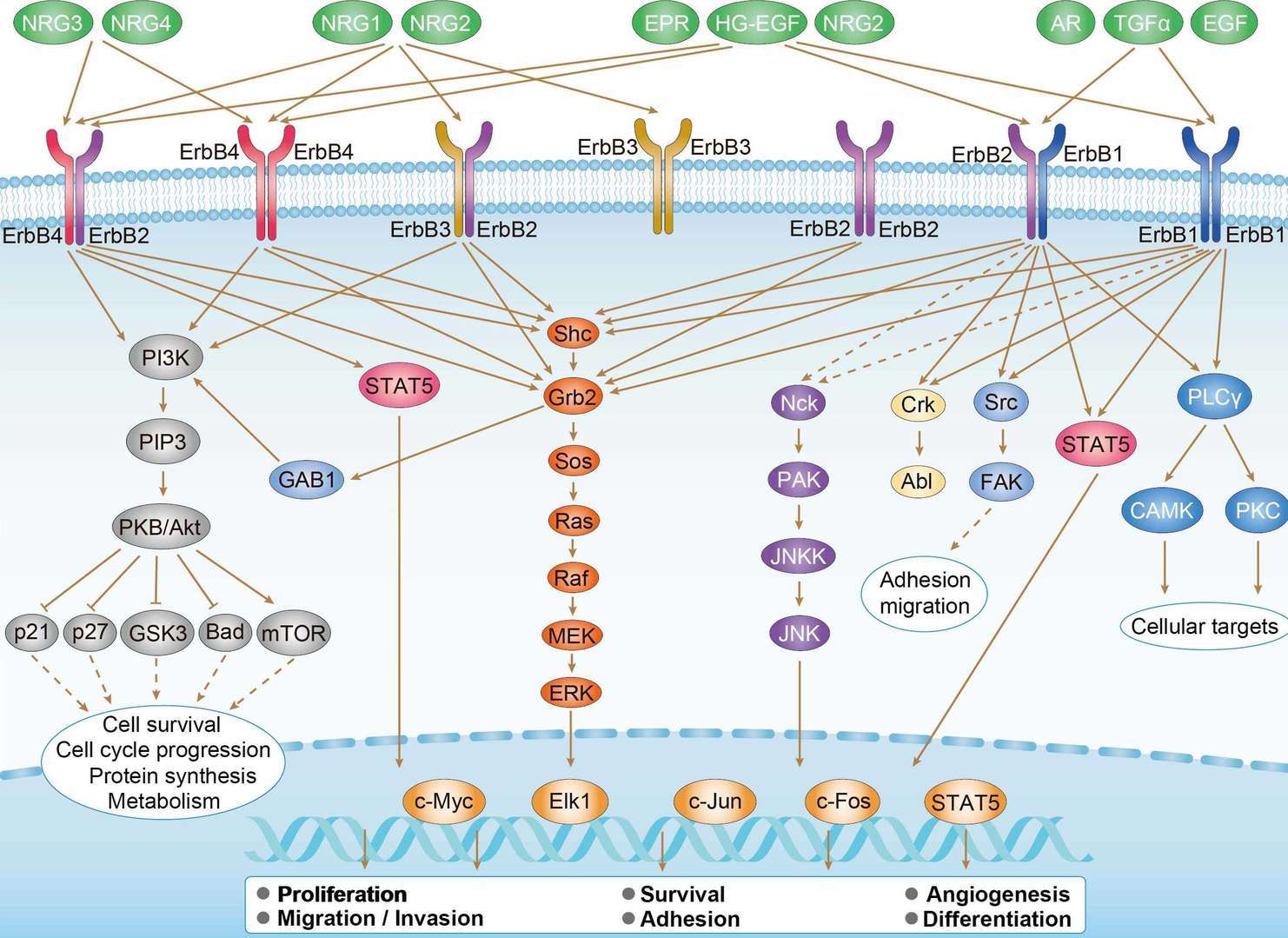 ErbB Signaling Pathway
ErbB Signaling Pathway
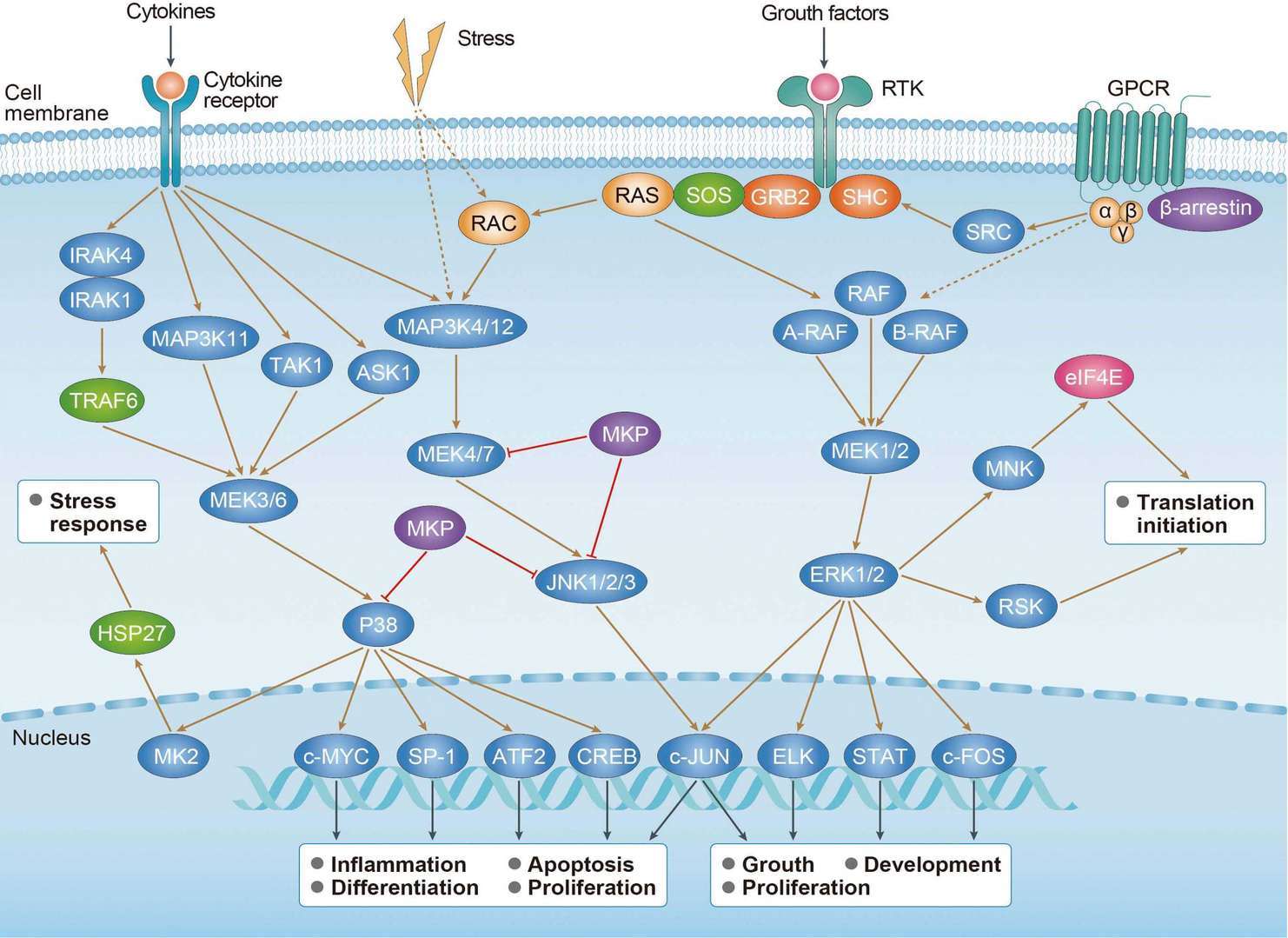
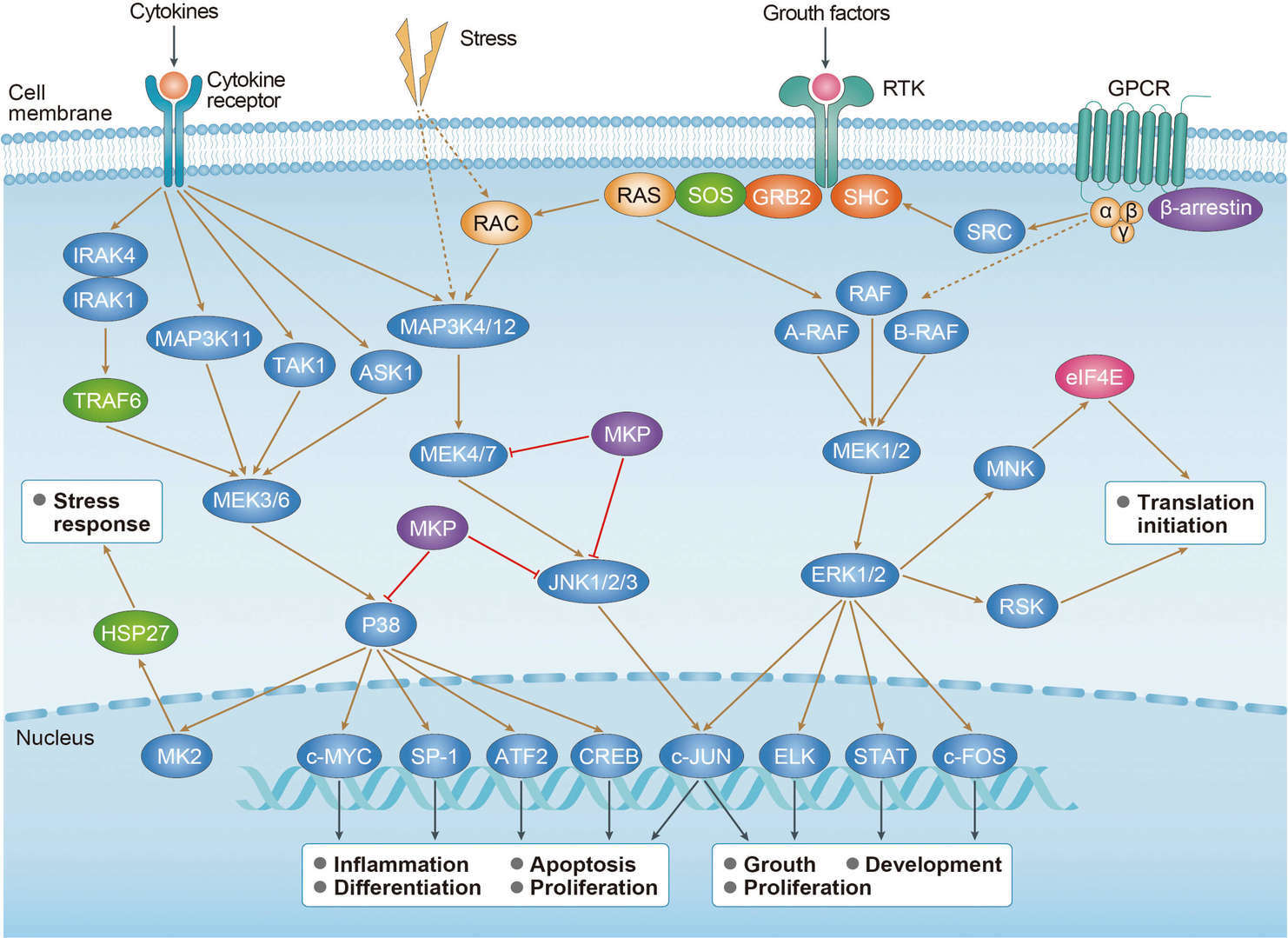 MAPK Signaling Pathway
MAPK Signaling Pathway