Tositumomab Overview
Introduction of Tositumomab
Tositumomab is a murine IgG2a lambda monoclonal antibody directed against CD20 molecular, which is a transmembrane phosphoprotein expressed on pre-B-lymphocytes and mature B-lymphocytes. In combination with a radioactive atom Iodine - 131, tositumomab has been used for the treatment of patients with CD20 positive, relapsed or refractory, low-grade, follicular, or transformed non-Hodgkin's lymphoma who have progressed during or after rituximab therapy, including patients with rituximab-refractory non-Hodgkin's lymphoma.
Mechanism of Action of Tositumomab
Tositumomab is a protein drug that usually radiolabeled with iodine I 131, which has potential antineoplastic activity. Tositumomab is a monoclonal antibody that could bind to the CD20 surface membrane antigen expressed on the B-lymphocytes. Binding of tositumomab to CD20 appears to induce apoptosis and may stimulate antitumoral cell-mediated and/or antibody-dependent cytotoxicity. In addition, when the antibody attaches to the CD20 antigens, the radiation from radioactive elements iodine I 131 is delivered directly to the targeted cells killing the targeted cells as well as cancer cells in the immediate area by ionizing radiation. Besides, a mechanism of potential vaccine-like effect leading to adaptive immunity against cells that survive initial treatment is also suggested.
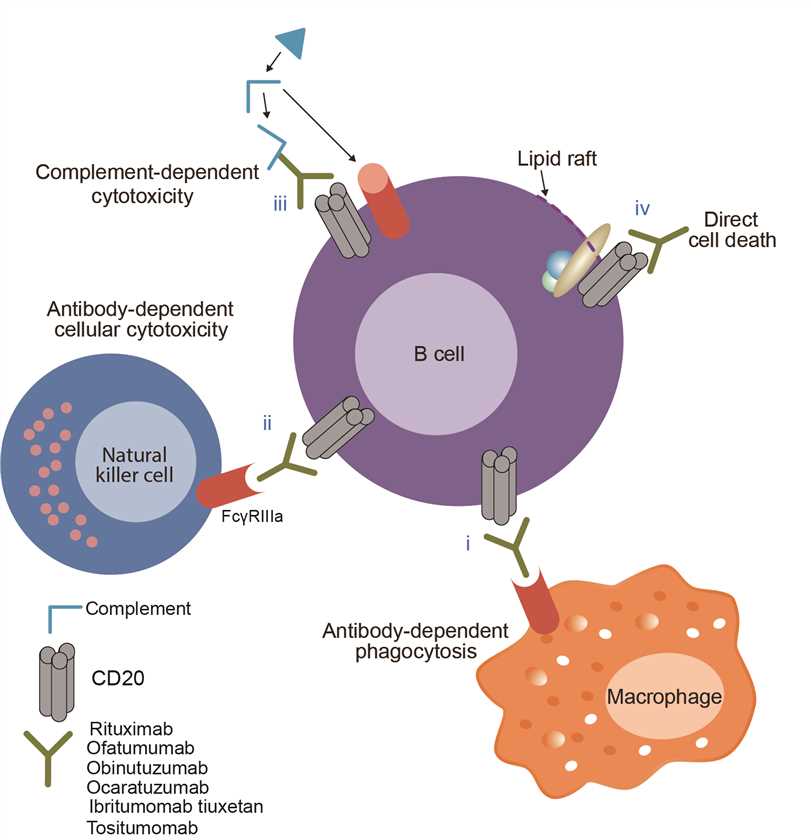
Fig.1 Mechanism of action of Tositumomab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



