Varlilumab Overview
Introduction of Varlilumab
Varlilumab (CDX-1127), a human IgG1κ monoclonal antibody, targets CD27 receptor through reacting with the ligand binding site of CD27. CD27 is a member of the tumor necrosis factor receptor superfamily and expressed on unstimulated T lymphocytes, serving as a potent costimulatory molecule. It has been found that CD27 induces a series of T-cell reactions including T-cell activation, proliferation, survival, and maturation of effector capacity and T-cell memory together with its ligand CD70, which is transiently expressed on antigen-presenting cells. Besides, the interaction of CD27 and CD70 also stimulate B-cell proliferation, generation of plasma cells, production of immunoglobulin and B-cell memory, and induction of the cytolytic activity of natural killer (NK) cells. Moreover, CD27 is considered to function on tumor expansion and activation as it also expressed by regulatory T cells (Tregs), a cell that is associated with the suppression of antitumor immunity. The drug, is designed for immunotherapy for patients with solid tumors and hematologic malignancies. Varlilumab is currently conducted with two trails. One is the phase 1B clinical trial for advanced breast or ovarian cancer in combination with ONT-10. Another is the phase 1/2 trial for advanced refractory solid tumors together with anti-PD-1 nivolumab.
Mechanism of Action of Varlilumab
CD27 is known as a co-stimulatory molecule on T cells and exerts biological functions through binding with its ligand CD70. However, the interaction of CD27 and CD70 also can induce some refractory autoimmune diseases. Varlilumab is designed as an anti-CD27 antibody that inhibits the binding of CD27 to CD70, helping activate the T-cells. Varlilumab is also shown direct therapeutic effects against tumors as CD27 is often highly expressed in Human B and T cell lymphomas. Thus, varlilumab may exert its function via two independent mechanisms. On the one hand, Varlilumab serves as a substitution for CD70 and activates T cells by binding to CD27, which results in a weak immune response becoming a strong response that can last for a long time. On the other hand, Varlilumab exerts direct anti-tumor effect on tumors that expressed high level of CD27. When Varlilumab directly binds to CD27 on the surface of tumor cells, the mechanism known as antibody dependent cellular cytotoxicity (ADCC) will be initiated, triggering the destruction of tumor cells.
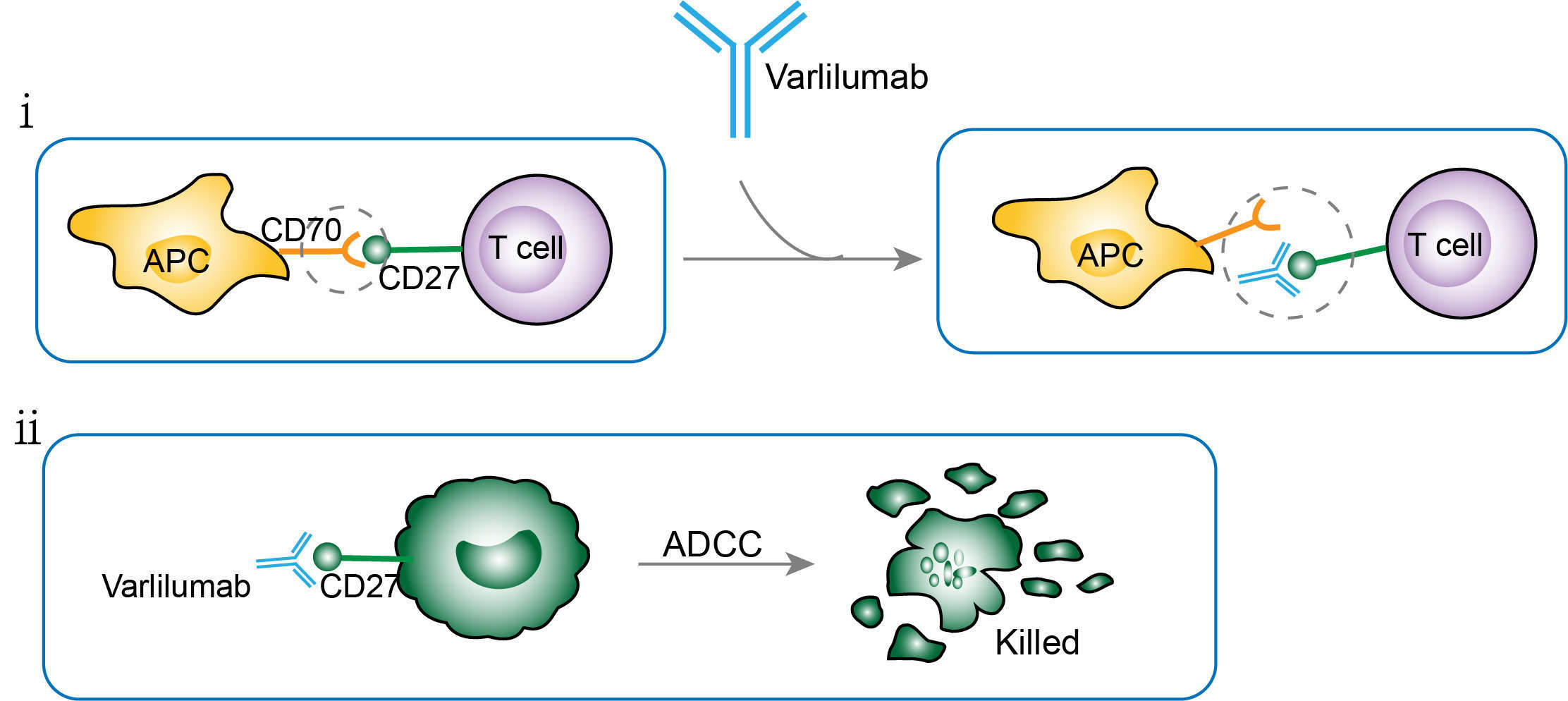 Fig.1 Mechanism of action of Varlilumab
Fig.1 Mechanism of action of Varlilumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

