Vesencumab Overview
Introduction of Vesencumab
The Anti-Human NRP1 Recombinant Antibody (Vesencumab) is a recombinant monoclonal antibody against Neuropilin-1 (NRP-1), a co-receptor involved in tumor angiogenesis and immune regulation. Vesencumab is a humanized IgG1 antibody that works to prevent angiogenesis and the growth of new blood vessels, which is critical for solid tumor growth and dissemination. It functions by mainly binding to NRP-1, preventing it from converting into Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2), and thereby interfering with the VEGF-mediated signaling pathway responsible for endothelial cell growth and the development of blood vessels. This process is essential for inhibiting tumor-associated blood vessel formation that provides the necessary oxygen and nutrients to enlarge tumors. The antibody can be used to suppress tumor growth, according to preclinical models. Vesencumab showed exceptional tumor inhibition in the SUM159 xenograft model, and after seven weeks of treatment, the Vesencumab group had tumors 12.8 times smaller than the control group. This finding suggests that Vesencumab effectively blocks tumor growth and angiogenesis, potentially shrinking the size and spread of the tumor.
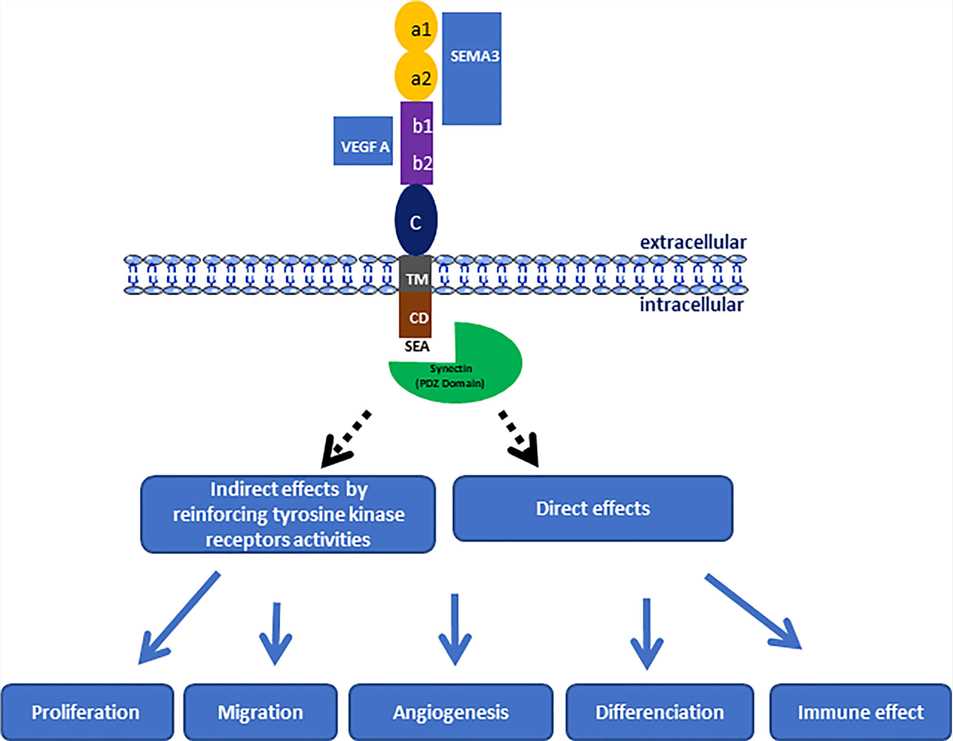 Figure 1. Schematic Representation of NRP-1 Structure.1,3
Figure 1. Schematic Representation of NRP-1 Structure.1,3
Vesencumab is a promising anti-angiogenesis and anti-tumor drug, especially for the treatment of metastatic solid tumors such as ovarian cancer and others. The clinical trials have demonstrated that Vesencumab could be used in combination with already approved anti-VEGF therapies such as bevacizumab to further boost anti-angiogenic properties, and therefore allow for more promising regimens. But trials of this combination were halted because of severe nephrotoxic reactions, highlighting the importance of further study on the safety and dosage of this treatment approach.
The Mechanism of Vesencumab Action
Vesencumab functions by directly targeting NRP-1, an important cell surface receptor for angiogenesis and immunity. Vesencumab binding to NRP-1 stops it from interacting with VEGFR-2, a transcription factor involved in the signaling cascade that initiates new blood vessels. This destabilization of VEGF-dependent signaling results in:
- Tumor vascularisation inhibition: By inhibiting the signaling pathway between NRP-1 and VEGFR-2, Vesencumab prevents endothelial cells from being activated and forming blood vessels in tumors, restricting growth.
- Reduced tumor expansion: Tumors without new blood vessels are deprived of nutrients and oxygen needed for survival and proliferation.
- Improved anti-tumor activity: When combined with other anti-VEGF drugs, Vesencumab has demonstrated the synergistic enhancement of anti-angiogenic activities, which can lead to improved therapeutic efficacy.
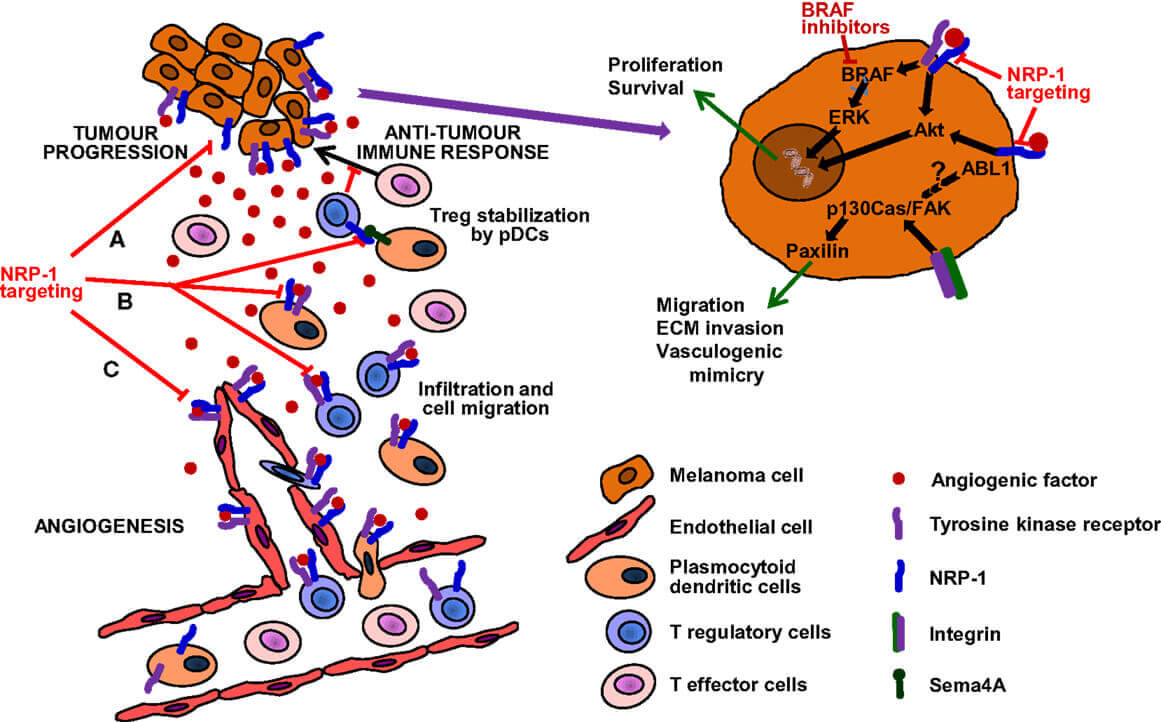 Figure 2. Targeting of NRP-1 in the Treatment of Melanoma.2,3
Figure 2. Targeting of NRP-1 in the Treatment of Melanoma.2,3
This system plays an even more important role in metastatic cancers, where tumors that are able to develop new blood vessels are often connected to the proliferation and invasion of distant organs. By affecting NRP-1, Vesencumab directly interferes with a critical mechanism for tumor metastasis.
The Clinical Applications of Vesencumab
Vesencumab is also under clinical trial to determine its efficacy as a treatment for other metastatic solid tumors. These are cancers that are especially hard to cure because they're so aggressive and rapidly develop new blood vessels that feed the tumor. These are some of the major clinical applications in development:
- Ovarian Cancer: The blocking effect of Vesencumab on NRP-1/VEGFR-2 interaction could be potentially useful for patients with ovarian cancer, where angiogenesis plays a critical role in the progression and spread of cancer.
- Other Solid Tumors: Vesencumab is also in clinical trials for other advanced cancers such as breast, non-small cell lung, and colon cancers, where angiogenesis is the primary cause of tumor expansion and metastasis.
- Use in Combination with Anti-VEGF Agents: Vesencumab can be combined with current therapy including bevacizumab (an anti-VEGF monoclonal antibody) to enhance anti-angiogenic activity for an effective treatment combination.
The Concerns and Future of Vesencumab
Preclinical results have been encouraging, but the clinical trial of Vesencumab has been hampered by toxicity concerns. Acute side effects—including infusion reactions, fatigue, pruritus, myalgia, and thrombocytopenia—were noted during early clinical trials. These adverse effects are troubling, and highlight the need for ongoing monitoring of patients receiving Vesencumab. Moreover, the Vesencumab/bevacizumab combination caused severe nephrotoxic side effects and had to be abandoned for certain trials. Such concerns call for further refinement of the dosing schedule and combination approach to maintain patient safety and achieve therapeutic efficacy. Although Vesencumab has had a lot of early clinical trial setbacks, it still holds promise as a cancer treatment. Ongoing research is focused on:
- Optimizing dosing: Adjusting the dose of Vesencumab can lead to increased tolerability and fewer side effects.
- Testing it in more cancers: It is being tested for different types of cancers, and its combination therapy with Vesencumab could help define the right patient populations.
- Exploring biomarkers for patient selection: In order to maximize Vesencumab's efficacy, researchers are trying to figure out how to target patients who might be most susceptible to its benefits.
Since it interferes with a major pathway in tumor angiogenesis, Vesencumab has promise as a treatment for many types of solid tumors, especially those that are often highly metastasized. But there are still safety and efficacy issues, and more research is needed before this new treatment could be exploited.
What We Provide
Anti-Human NRP1 Recombinant Antibody (Vesencumab)
We provide high-quality Vesencumab for use in WB, FuncS, IF, Neut, ELISA, FC, IP and most other immunological methods. The product is for lab research use only, not for diagnostic, therapeutic, or any in vivo human use.
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Human
- Derivation
- Human
- Type
- IgG1 - kappa
- Specificity
- Tested positive against native human antigen.
- Species Reactivity
- Human
- Applications
- Suitable for use in WB, FuncS, IF, Neut, ELISA, FC, IP and most other immunological methods.
- CAS
- 1205533-60-3
- Generic Name
- Vesencumab
- MW
- 145.0 kDa
- Related Disease
- Solid tumors
- Douyère, Manon, Pascal Chastagner, and Cédric Boura. "Neuropilin-1: a key protein to consider in the progression of pediatric brain tumors." Frontiers in Oncology 11 (2021): 665634.
- Graziani, Grazia, and Pedro M. Lacal. "Neuropilin-1 as therapeutic target for malignant melanoma." Frontiers in oncology 5 (2015): 125.
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

