
Autophagy
Autophagy is a fundamental cellular process responsible for the degradation and recycling of cellular components. This process is essential for maintaining cellular homeostasis, responding to stress, and regulating cell survival. It involves the lysosomal degradation of damaged organelles, misfolded proteins, and other cellular debris, which are critical for cellular repair and regeneration. The regulation of autophagy is complex and involves a variety of signaling pathways and protein complexes. Among the most significant are AMP-activated protein kinases (AMPK), the ATG (autophagy-related) family of proteins, mTOR complexes, and Rab proteins. Each plays a distinct role in modulating autophagy, responding to cellular energy levels, nutrient availability, and stress signals.
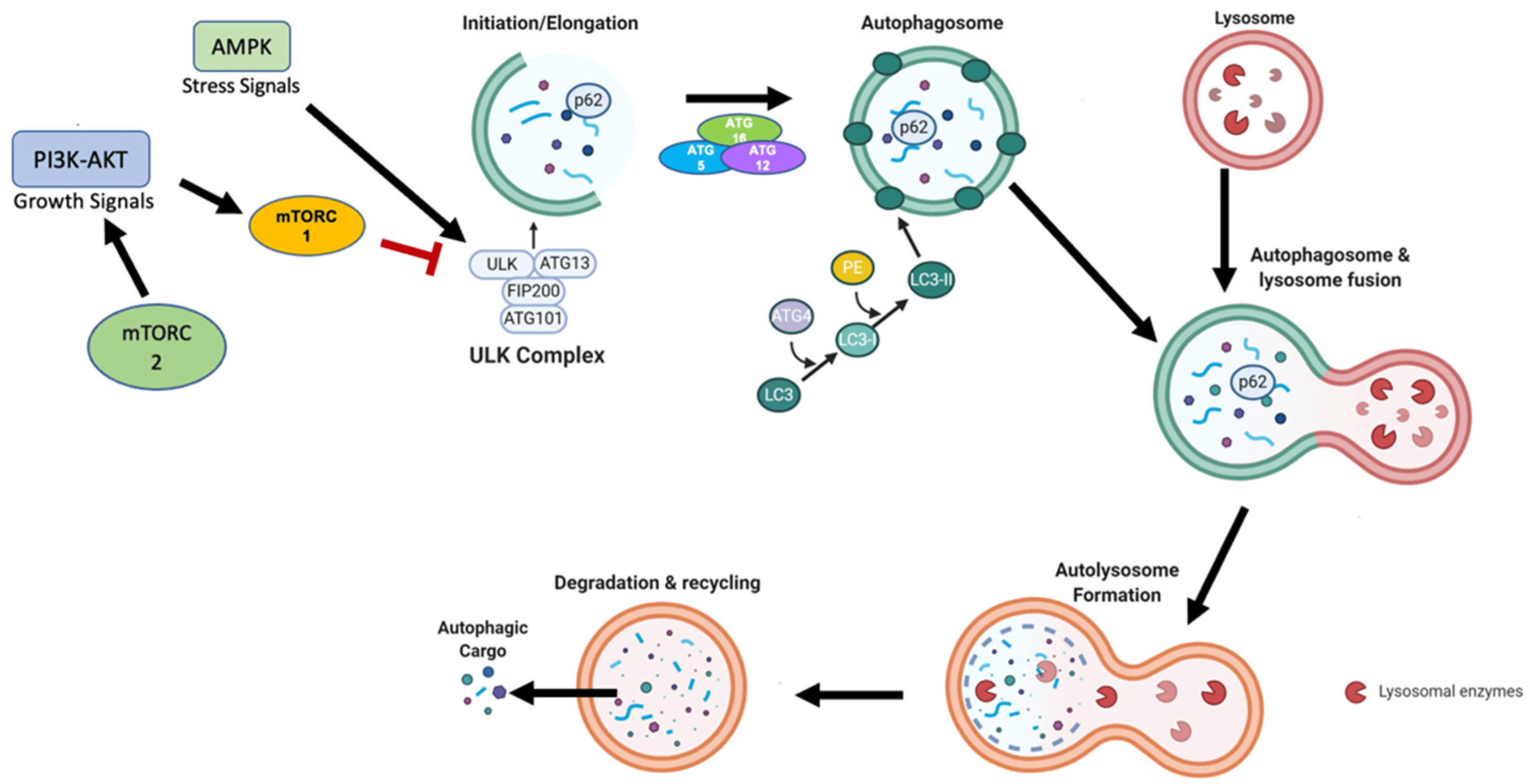 Figure 1 Primary mechanism of autophagy. (Finnegan, 2022)
Figure 1 Primary mechanism of autophagy. (Finnegan, 2022)
AMP-activated Protein Kinases (AMPK)
AMPK serves as a critical energy sensor within the cell, playing a central role in maintaining energy homeostasis. Structurally, AMPK is a heterotrimeric complex composed of a catalytic α subunit and regulatory β and γ subunits, each of which is crucial for the enzyme's stability and activity. The α subunit contains the kinase domain responsible for the phosphorylation of various substrates. The β subunit serves primarily as a scaffold, holding the complex together and interacting with the α and γ subunits; it also contains a carbohydrate-binding module which may help in sensing cellular energy levels through glycogen content. The γ subunit has four cystathionine beta-synthase (CBS) domains, which bind AMP or ATP, enabling AMPK to sense shifts in the cellular AMP to ATP ratio.
AMPK is activated by increases in intracellular AMP levels, which occur during energy depletion (e.g., exercise, hypoxia). Binding of AMP to the γ subunit causes a conformational change that protects the α subunit's threonine-172 (Thr172) from dephosphorylation and makes it more accessible to upstream kinases like LKB1 (liver kinase B1), which phosphorylates and activates it. Interestingly, recent studies suggest that ADP, like AMP, can also bind to the γ subunit and promote AMPK activation, albeit less effectively than AMP.
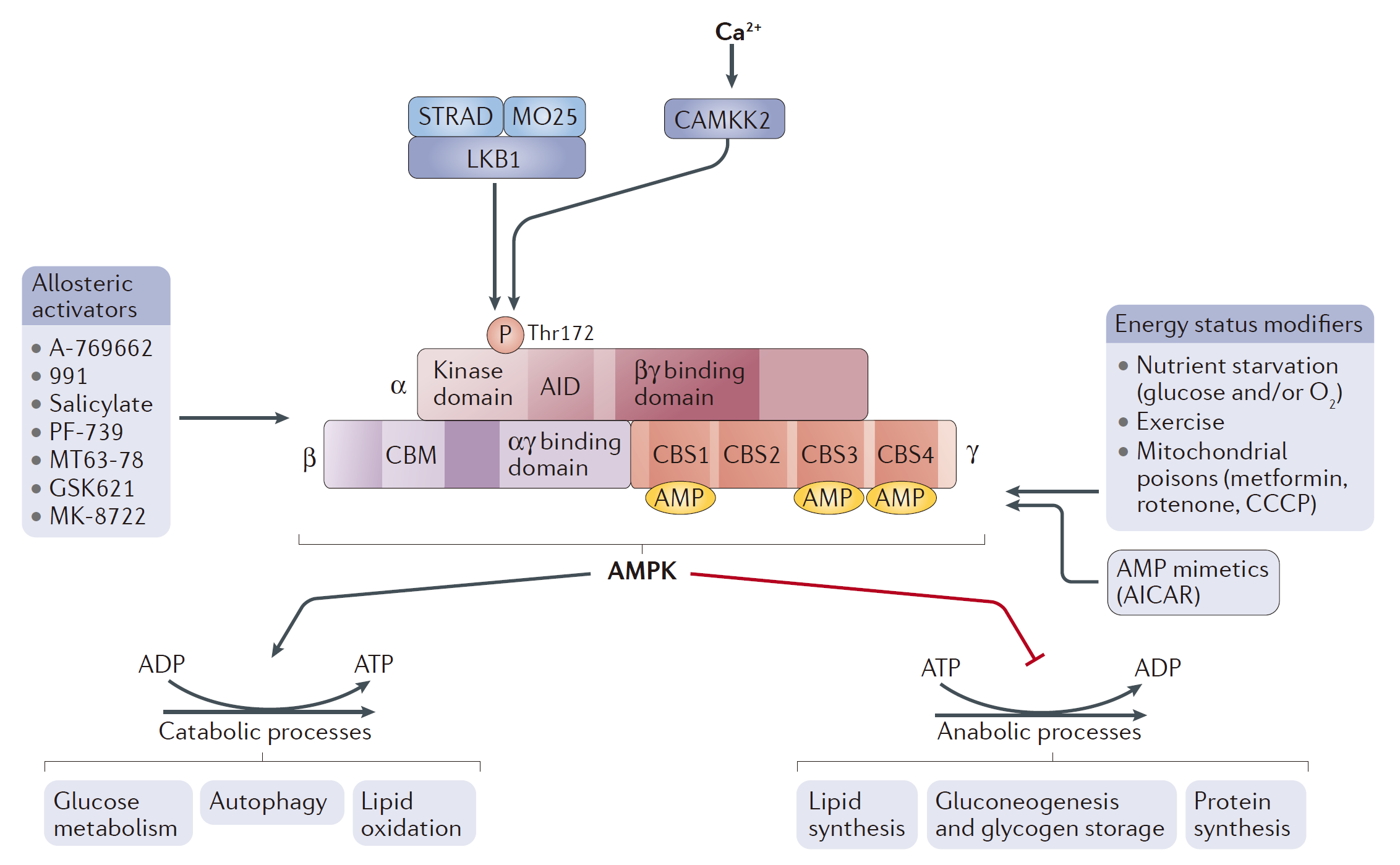 Figure 2 AMPK structure and activation. (Herzig, 2018)
Figure 2 AMPK structure and activation. (Herzig, 2018)
Once activated, AMPK initiates a broad response to restore energy balance by enhancing catabolic pathways that generate ATP (such as fatty acid oxidation and glycolysis) and inhibiting anabolic processes (such as protein synthesis and lipogenesis) that consume ATP. For instance, AMPK directly phosphorylates acetyl-CoA carboxylase (ACC), inhibiting fatty acid synthesis and promoting fatty acid oxidation—a rapid response to immediate energy demands. Moreover, AMPK influences other key regulators of metabolism, including the mTOR pathway. By phosphorylating TSC2 and Raptor, two components of the mTOR signaling pathway, AMPK inhibits mTORC1, which is a potent anabolic regulator that, when active, promotes protein synthesis and cell growth. This inhibition is vital during low energy states as it conserves energy and shifts cellular priorities towards survival rather than growth.
ATG Family
The ATG family of proteins is instrumental in the autophagic process, with over 30 ATG proteins identified as playing a role in autophagy in yeast, many of which have mammalian counterparts. These proteins orchestrate the formation and elongation of the autophagosome. Key among these proteins is the ATG1/ULK1 complex, which is one of the initial activators of the autophagic process. This complex is regulated by nutrient availability through the actions of AMPK and mTOR, making it a critical nexus point in autophagy initiation. Other important ATG proteins include ATG9, which is involved in membrane delivery to the autophagosome, and the ATG12-ATG5-ATG16L complex, which is essential for the expansion of the autophagosome. The lipidation of ATG8/LC3, facilitated by this complex, is a hallmark of autophagosome maturation, serving as a key indicator of autophagic activity. The coordinated action of the ATG proteins ensures the efficient turnover of cellular components, contributing to cell survival and adaptation to metabolic stress.
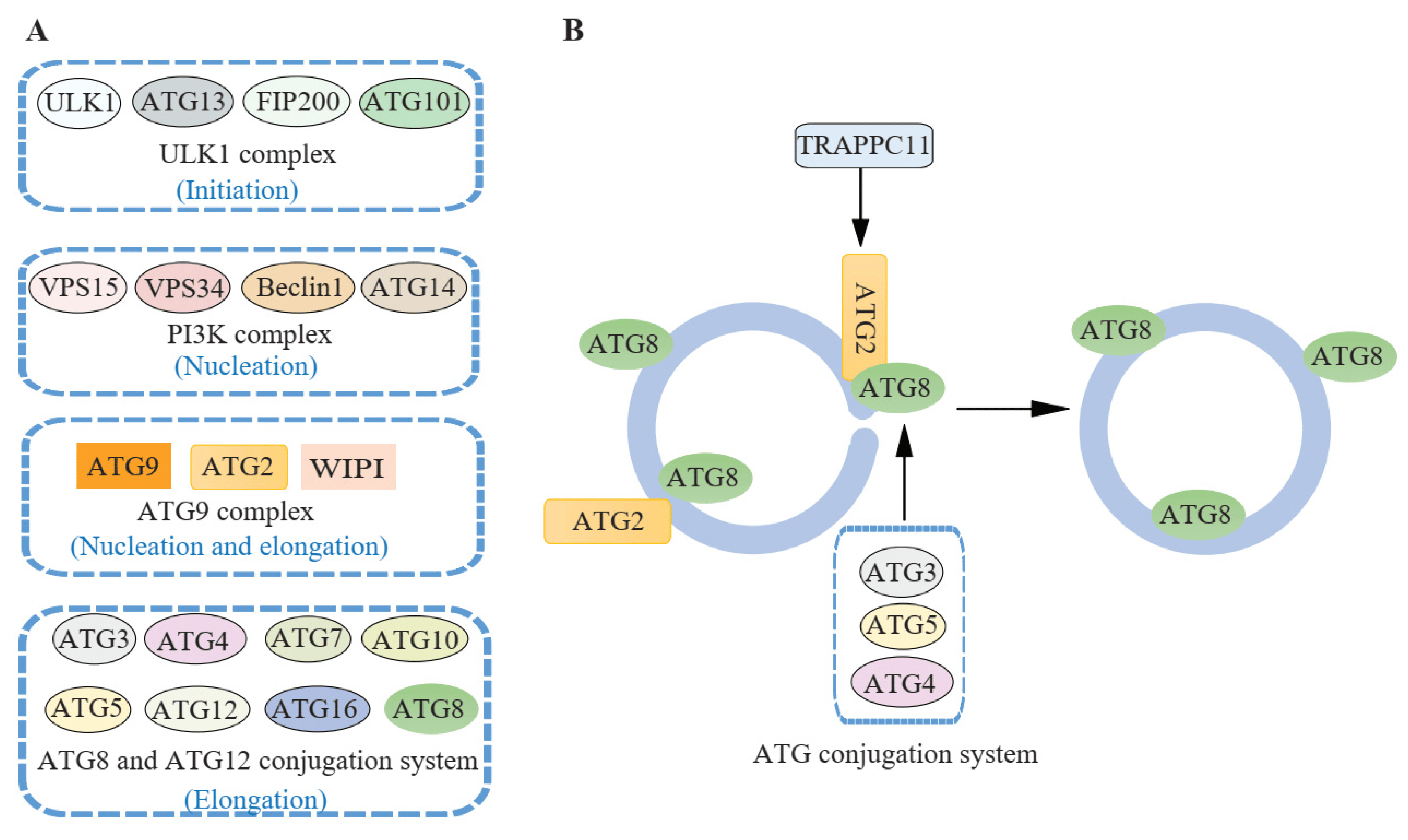 Figure 3 ATG proteins involved in autophagosome closure. (Jiang, 2021)
Figure 3 ATG proteins involved in autophagosome closure. (Jiang, 2021)
The regulation of ATG proteins is meticulously coordinated, often involving post-translational modifications such as phosphorylation, ubiquitination, and conjugation reactions. These modifications ensure that autophagy is responsive to cellular conditions and that the autophagic machinery is assembled only when required, preventing unnecessary or excessive autophagic activity. The function of ATG proteins extends beyond the canonical autophagy pathway to include roles in non-autophagic processes such as secretion and the immune response. For instance, certain ATG proteins are involved in the unconventional secretion of cytokines and the presentation of endogenous antigens on MHC molecules, highlighting their versatility and importance in cellular physiology.
mTOR Complexes
The mechanistic target of rapamycin (mTOR) signaling pathway is a crucial regulator of cell growth, proliferation, and survival. It responds to a variety of environmental cues, including nutrient levels, growth factors, and cellular energy status. The mTOR protein forms two distinct multi-protein complexes known as mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2), each of which has unique components and functions.
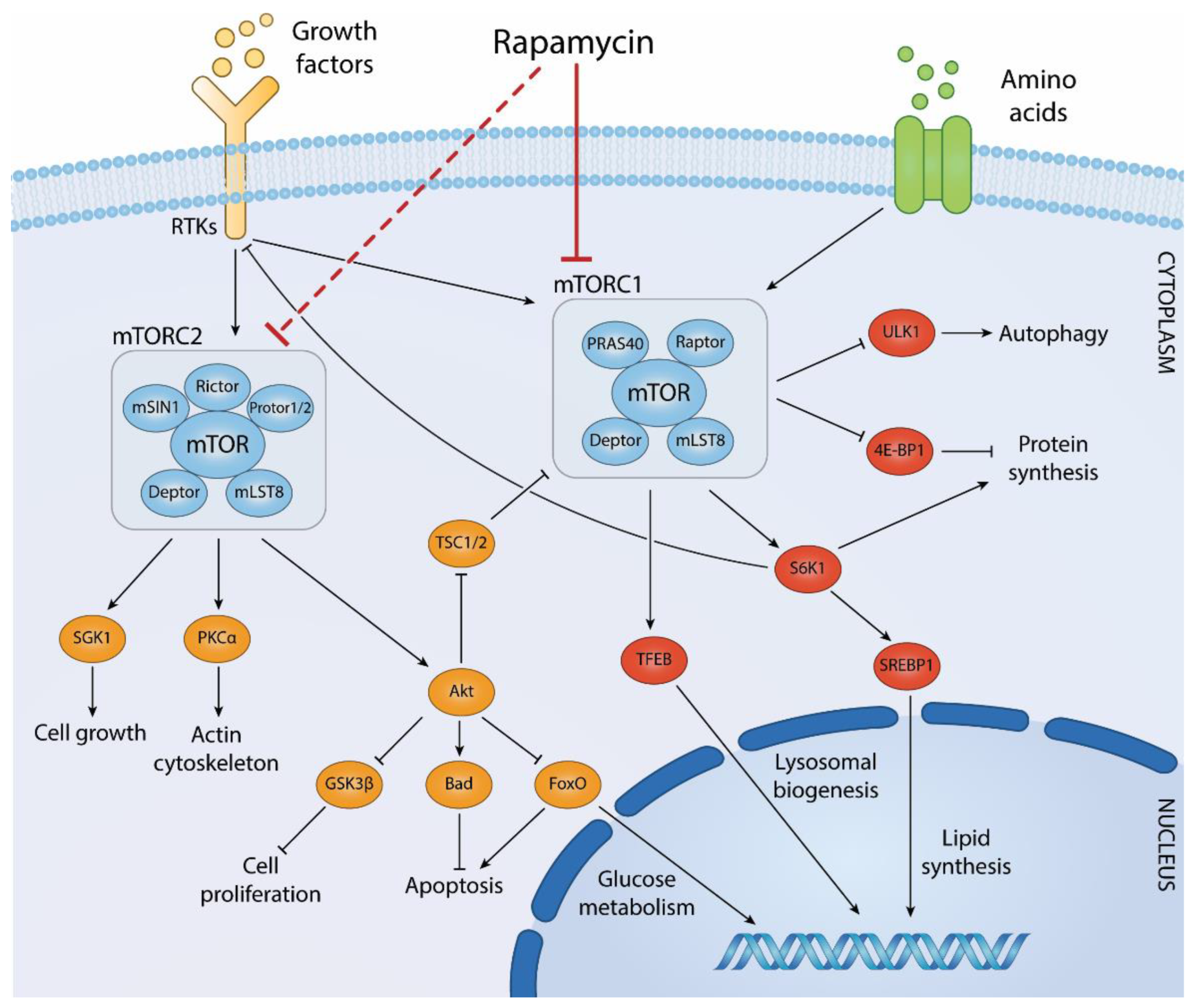 Figure 4 Biological processes regulated by mTOR complexes and downstream effector pathways. (Centonze, 2022)
Figure 4 Biological processes regulated by mTOR complexes and downstream effector pathways. (Centonze, 2022)
mTORC1 is the more extensively studied of the two complexes and plays a key role in the negative regulation of autophagy. This complex consists of mTOR itself, regulatory-associated protein of mTOR (RAPTOR), mammalian lethal with SEC13 protein 8 (mLST8), DEP domain-containing mTOR-interacting protein (DEPTOR), and the proline-rich AKT substrate 40 kDa (PRAS40). mTORC1 is sensitive to nutrient availability, particularly amino acids, and growth factors. Under conditions of nutrient sufficiency, mTORC1 is activated and promotes protein synthesis and cell growth by phosphorylating key downstream targets such as S6 kinase (S6K) and the eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). These actions inhibit the process of autophagy by maintaining cell growth and suppressing catabolic processes, including the degradation of cellular components. One critical mechanism through which mTORC1 regulates autophagy is its interaction with the ULK1 (Unc-51 like autophagy activating kinase 1) complex, which is vital for the initiation of autophagy. In nutrient-rich conditions, mTORC1 phosphorylates ULK1 and its associated protein ATG13, preventing the ULK1 complex from initiating autophagy. Conversely, nutrient starvation leads to the inhibition of mTORC1, derepressing ULK1 and promoting the autophagic response.
mTORC2 is less well understood compared to mTORC1 but is known to play a role in controlling cell survival, cytoskeletal dynamics, and lipid metabolism. This complex includes mTOR, rapamycin-insensitive companion of mTOR (RICTOR), mLST8, DEPTOR, and mSin1. Unlike mTORC1, mTORC2 is not directly responsive to nutrients but is regulated by growth factors and insulin. mTORC2 phosphorylates and activates AKT, a serine/threonine kinase involved in promoting cell survival and growth. Activation of AKT by mTORC2 leads to the phosphorylation of a range of downstream targets that support cell proliferation and survival. Additionally, mTORC2 regulates the actin cytoskeleton, which is crucial for maintaining cell shape, motility, and integrity. The roles of mTORC1 and mTORC2 in regulating cellular processes underscore the complexity of the mTOR pathway as a whole. Disruptions in mTOR signaling are implicated in a variety of diseases, including cancer, obesity, type 2 diabetes, and neurodegenerative disorders. Consequently, the mTOR pathway is a significant target for therapeutic intervention, with several mTOR inhibitors currently used or being tested in clinical settings to treat these conditions.
Rab Proteins
Rab proteins are a large family of small GTPases that regulate various aspects of membrane trafficking, including vesicle formation, vesicle movement along actin and tubulin networks, and membrane fusion. In the context of autophagy, Rab proteins play crucial roles in the maturation and trafficking of autophagosomes to lysosomes.
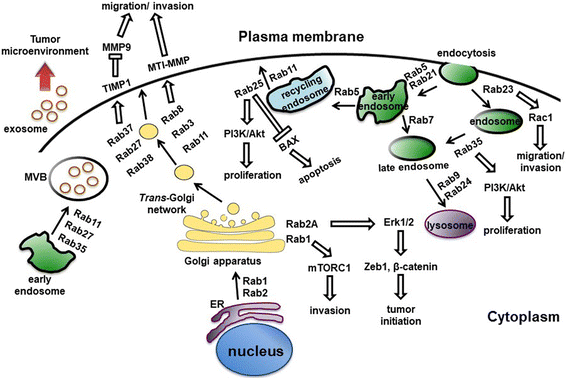 Figure 5 Rab proteins-mediated vesicular transport and signaling pathways. (Tzeng, 2016)
Figure 5 Rab proteins-mediated vesicular transport and signaling pathways. (Tzeng, 2016)
Within the context of autophagy, several Rab proteins have been identified as key regulators. Rab7, as previously mentioned, is one of the most well-studied in this process. It is essential for the late stages of autophagy, particularly in the maturation of autophagosomes and their subsequent fusion with lysosomes to form autolysosomes. Rab7 works by recruiting effector proteins that facilitate the tethering and fusion of membrane compartments. For example, it interacts with the HOPS complex, a multi-subunit tethering complex, which is crucial for mediating the fusion between autophagosomes and lysosomes. Another significant Rab protein is Rab5, which is involved in the early stages of autophagy. Rab5 is primarily known for its role in endocytosis but also contributes to the early endosomal stages of autophagosome formation. It promotes the fusion of vesicles to form early autophagic structures and can interact with VPS34, a class III PI3 kinase that is pivotal in initiating autophagy by producing phosphatidylinositol 3-phosphate (PI3P) on early endosomes and autophagic membranes.
The function of Rab proteins is tightly regulated by their ability to cycle between an active, GTP-bound state and an inactive, GDP-bound state. This cycle is modulated by two sets of regulatory proteins: guanine nucleotide exchange factors (GEFs), which promote the exchange of GDP for GTP, and GTPase-activating proteins (GAPs), which accelerate the hydrolysis of GTP to GDP. This cycling is crucial for the proper functioning of Rab proteins, as it allows them to interact dynamically with various effector proteins that dictate their specific roles in vesicle trafficking. Moreover, Rab proteins often work in concert with other Rab members to coordinate different stages of vesicle transport. For instance, Rab5 and Rab7 can act sequentially to control the progression from early to late stages in both endocytosis and autophagy. Such coordination ensures the continuity and efficiency of vesicle trafficking processes, vital for maintaining cellular homeostasis.
- Finnegan, Ryan M., et al. "Therapeutic potential for targeting autophagy in ER+ breast cancer." Cancers 14.17 (2022): 4289.
- Herzig, Sébastien, and Reuben J. Shaw. "AMPK: guardian of metabolism and mitochondrial homeostasis." Nature reviews Molecular cell biology 19.2 (2018): 121-135.
- Jiang, Wenyan, et al. "Key regulators of autophagosome closure." Cells 10.11 (2021): 2814.
- Centonze, Giorgia, et al. "ROCK ‘n TOR: an Outlook on keratinocyte Stem Cell Expansion in Regenerative Medicine via protein kinase Inhibition." Cells 11.7 (2022): 1130.
- Tzeng, Hong-Tai, and Yi-Ching Wang. "Rab-mediated vesicle trafficking in cancer." Journal of biomedical science 23 (2016): 1-7.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
