
CAR-T Cell Therapy Targets
Chimeric antigen receptor (CAR)-T cell therapy stands out from traditional cancer treatments, such as nonspecific drugs and monoclonal antibodies, by employing the immune system's T cells to directly recognize and eliminate tumor cells. CAR-T cell therapy involves isolating T cells from a patient, genetically modifying them to express CARs that target tumor-associated antigens, and then reinfusing these engineered T cells back into the patient. These CAR-T cells are then able to specifically bind to antigens present on the surface of tumor cells and induce their destruction. This therapy has achieved significant success, particularly in the treatment of various blood cancers, by targeting specific antigens like CD19 and B-cell maturation antigens (BCMA), leading to its approval by regulatory bodies like the Food and Drug Administration (FDA) for certain leukemia and lymphomas.
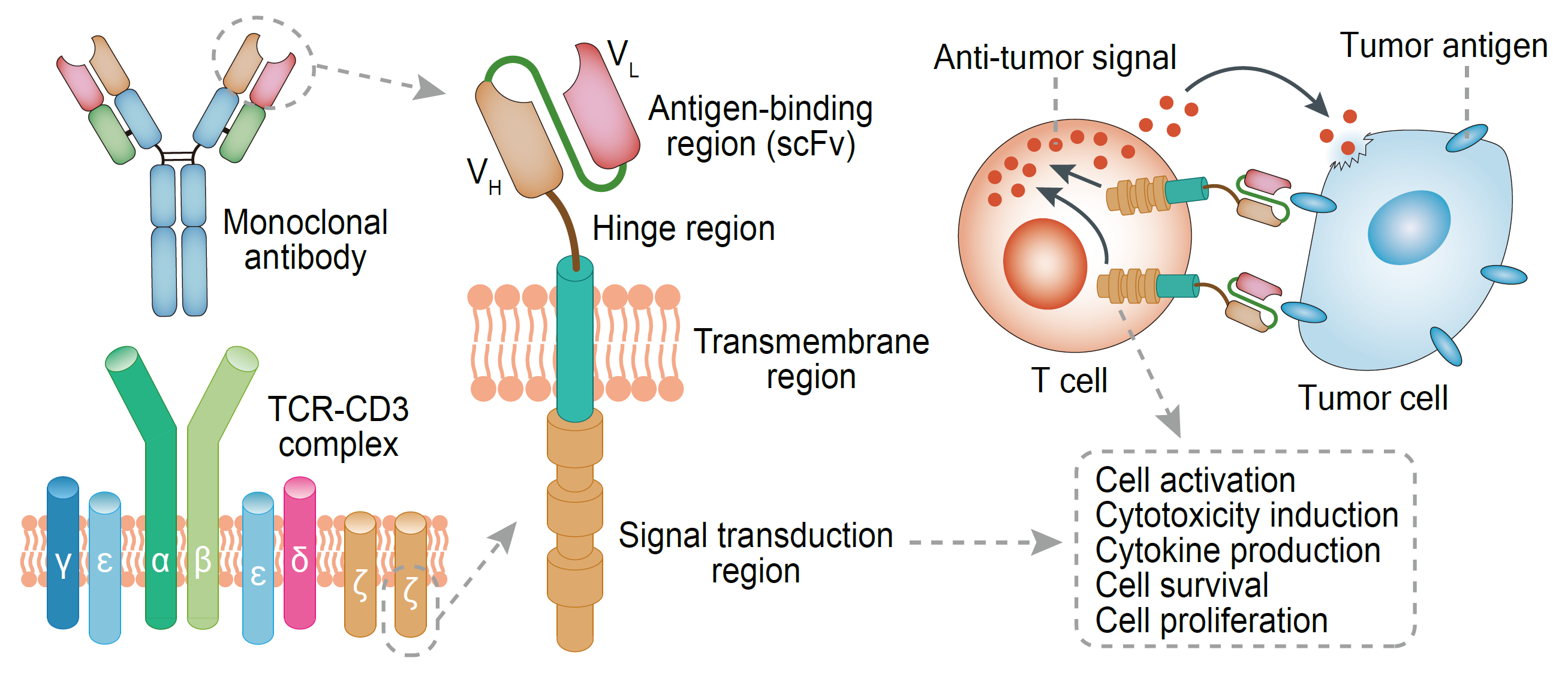 Figure 1 Structural and Functional Basis of chimeric antigen receptor (CAR).
Figure 1 Structural and Functional Basis of chimeric antigen receptor (CAR).
Representative Targets for CAR-T Cell Therapy
CD19
CD19 is a transmembrane protein that plays a crucial role in the immune system, particularly in the development, function, and regulation of B cells. It is expressed on the surface of B cells from early stages of development until their maturation into plasma cells, but it is not present on stem cells or other non-lymphoid cells. This makes CD19 an excellent target for immunotherapy, especially in B cell malignancies such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). Several CD19-targeted CAR-T cell therapies have received FDA approval: Kymriah (tisagenlecleucel) was the first CAR-T cell therapy approved by the FDA in 2017 for the treatment of patients up to 25 years old with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse. It is also approved for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy; Yescarta (axicabtagene ciloleucel) has been approved for adult patients with certain types of non-Hodgkin lymphoma (NHL) who have relapsed or are refractory after two or more lines of systemic therapy; Breyanzi (lisocabtagene maraleucel) has been approved for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma; Tecartus (brexucabtagene autoleucel) has been approved for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
-2.webp)


BCMA
BCMA is a member of the TNF receptor superfamily and interacts with two known ligands, APRIL (a proliferation-inducing ligand) and BAFF (B-cell activating factor), which promote B-cell survival and proliferation. The expression of BCMA increases as B cells mature, with the highest levels observed on plasma cells, the end stage of B-cell differentiation. In the context of cancer, especially multiple myeloma, BCMA aids in the survival and growth of malignant plasma cells. Therefore, targeting BCMA presents a promising approach for treating B-cell malignancies. Abecma (idecabtagene vicleucel) was the first BCMA-directed CAR-T cell therapy approved by the FDA for the treatment of relapsed or refractory multiple myeloma in adults who have received at least four prior lines of therapy. Carvykti (ciltacabtagene autoleucel) is another BCMA-targeted CAR-T cell therapy approved for the treatment of adults with relapsed or refractory multiple myeloma after four or more prior lines of therapy.
CD22
CD22 is a sialic acid-binding Ig-like lectin (Siglec) primarily expressed on the surface of mature B cells and to a lesser degree on some early B-cell precursors. Functioning as an inhibitory receptor, CD22 regulates B-cell activation and signal transduction, playing a pivotal role in maintaining immune homeostasis and preventing overactivation of the immune system. This receptor's expression pattern makes it an attractive target for therapeutic interventions in B-cell malignancies, such as certain types of leukemia and lymphoma. In the context of chimeric antigen receptor (CAR) T-cell therapy, CD22 has emerged as a promising target alongside the more widely recognized CD19. CAR-T cell therapies that target CD22 have shown considerable efficacy in patients who have relapsed or are refractory to CD19-targeted therapies, highlighting its importance as an alternative or complementary antigen target in the treatment of hematologic cancers. By engineering T cells to recognize and kill CD22-expressing B cells, researchers are expanding the arsenal against B-cell malignancies, offering new hope to patients with challenging-to-treat or relapsed diseases, thereby underscoring the significance of CD22 in advancing CAR-T cell therapeutic strategies.



CD20
CD20 is a cell surface protein primarily expressed on B cells, playing a crucial role in B-cell development and differentiation. As a member of the MS4A gene family, CD20 is involved in the regulation of intracellular calcium levels, which is essential for the activation and proliferation of B cells. Its expression from pre-B cells to mature B cells, but not on stem cells or plasma cells, makes it an ideal target for therapeutic interventions in B-cell malignancies. In the context of chimeric antigen receptor (CAR) T-cell therapy, CD20 has been exploited as a target antigen to direct CAR-T cells against CD20-expressing B-cell lymphomas and leukemias. CD20-targeted CAR-T cell therapy has shown promising results in treating certain types of non-Hodgkin lymphoma and chronic lymphocytic leukemia, offering a potent and targeted immunotherapy option for patients who are refractory to conventional treatments.

Full List of Targets for CAR-T Cell Therapy
Tested Data-Supported Products for CAR-T Cell Therapy Targets
- Van Cutsem, Eric, et al. "Gastric cancer." The Lancet 388.10060 (2016): 2654-2664.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
