
Gantenerumab Overview
Introduction of Gantenerumab
Gantenerumab is the first fully human IgG1κ monoclonal antibody designed to bind with subnanomolar affinity to a conformational epitope on β-amyloid (Aβ) fibrils. Gantenerumab was selected from a human phage display library and optimizes in vitro for binding with sub-nanomolar affinity to a conformation epitope expressed on Aβ fibrils. It passes the blood-brain barrier (BBB) and has a high capacity to specifically bind to cerebral amyloid plaques through recognizing both the N-terminal and central portions of Aβ. It is under investigation for the treatment of Dementia, Alzheimer’s Disease (AD), and Familial AD.
Mechanism of Action of Gantenerumab
The therapeutic rationale for gantenerumab is that it acts centrally to disassemble and degrade amyloid plaques by recruiting microglia and activating phagocytosis. It preferentially interacts with aggregated brain Aβ, both parenchymal and vascular, and elicits reduction of human Aβ deposits in AD brain. The exact mechanism of gantenerumab-mediated reduction of the amyloid burden is controversial. Several hypotheses concerning mechanisms of action in immunotherapy exist: 1) Plaque breakdown: Aβ plaques are destroyed through fragment crystallizable (Fc)-mediated phagocytosis by microglial cells. Gantenerumab is able to enter the brain and opsonize Aβ with resulting Fc receptor-mediated phagocytosis by microglia. 2) Peripheral sink: the formation of antigen-antibody complexes in the periphery sequesters amyloid away from the brain and prevents the deposition of new plaques. A further possibility supported by an increase in serum Aβ, most of which is bound to antibody, suggests that Aβ may also be removed from the brain directly into the blood by modifying the Aβ brain-blood equilibrium to enhance clearance of soluble Aβ. 3) Aggregation inhibitor: the formation of antigen-antibody complexes prevents amyloid from accumulating in plaques.
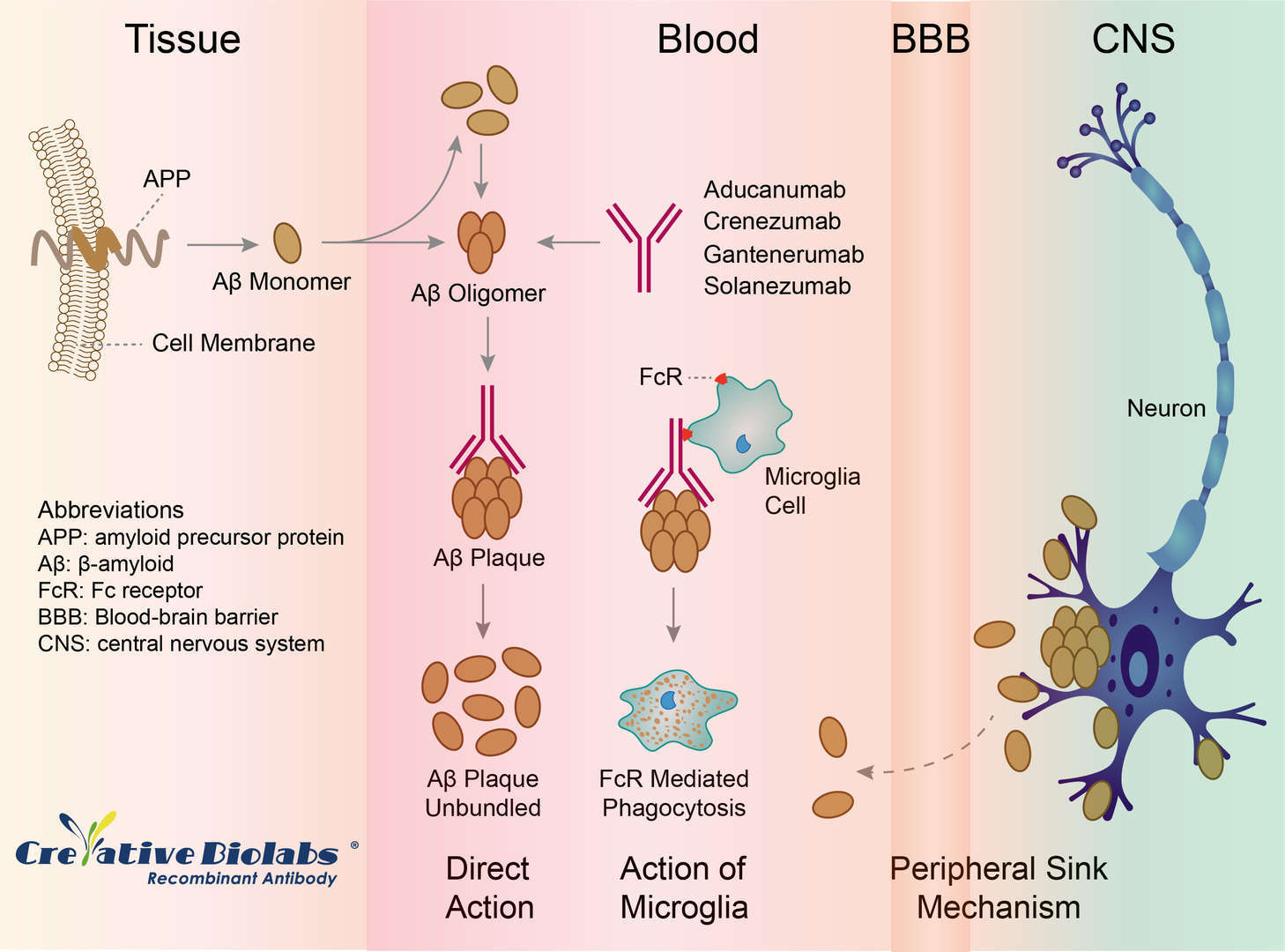 Fig.1 Mechanism of Action of Gantenerumab
Fig.1 Mechanism of Action of Gantenerumab
Clinical Projects of Gantenerumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03444870 | Not yet recruiting | Alzheimer Disease | Hoffmann-La Roche | February 23, 2018 |
| NCT03443973 | Not yet recruiting | Alzheimer's Disease | Hoffmann-La Roche | February 23, 2018 |
| NCT02051608 | Active, not recruiting | Alzheimer's Disease | Hoffmann-La Roche | January 31, 2014 |
| NCT01224106 | Active, not recruiting | Alzheimer's Disease | Hoffmann-La Roche | October 19, 2010 |
What We Provide
Therapeutic Antibody
Gantenerumab
We provide high-quality Gantenerumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Resource
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Gantenerumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

