Olokizumab Overview
Introduction of Olokizumab
Olokizumab (CDP6038), a humanized monoclonal antibody (mAb) specific for the interleukin-6 (IL-6) cytokine, is currently in development for the treatment of rheumatoid arthritis (RA). It targets the IL-6 cytokine rather than the receptor, and selectively blocks the final assembly of the signalling complex. In Phase I (healthy volunteers) and IIa (patients with RA on chemotherapy agent) clinical trials, olokizumab was well tolerated after intravenous and subcutaneous delivery with a median plasma half-life of approximately 31 days, 76% bioavailability and no apparent antidrug antibody-mediated clearance. Olokizumab also markedly reduced free IL-6 levels and suppressed C-reactive protein (CRP) up to 12 weeks after single-dose subcutaneous administration in patients with RA.
Mechanism of Action of Olokizumab
Interlukin-6 (IL-6) is a multifunctional cytokine with a central role in inflammation, infection responses, regulation of metabolic, regenerative, and neural processes. It has broad effect on cells of the immune system and is produced by T and B lymphocytes, fibroblasts, monocytes, keratinocytes, mesangial and endothelial cells, and several tumor cells. Normal physiological concentrations of IL-6 in human serum are relatively low, but rapidly elevated during infection, autoimmunity, and inflammation. IL-6 deficiency leads to impaired innate and adaptive immunity to viral, parasitic, and bacterial infection. In contrast, excessive production of IL-6 may leads to chronic autoimmune as well as inflammatory diseases such as psoriasis, rheumatoid arthritis, systemic lupus erythematosus, systemic juvenile idiopathic arthritis, Crohn’s disease, and tumorigenesis. Therefore, IL-6 has context-dependent pro- and anti-inflammatory properties and considered as a prominent target for clinical interventions. IL-6 has three distinct conserved epitopes: Site I, Site II, and Site III. IL-6, first form a complex with nonsignaling receptor, IL-6R (also known as gp80, CD126), through Site I and then form composite epitope known as Site II which interacts with cytokine-binding homology region of glycoprotein 130 (gp130) to constitute IL-6R:IL-6:gp130 trimer. Subsequent interaction of IL-6 Site III with gp130 immunoglobulin-like activation domain of two trimers form competent biologically active hexameric (ternary) signaling complex and activate Janus kinase (JAK) / signal transducer and activator of transcription-3 (STAT-3) pathway leading to inflammation. Olokizumab targets IL-6 itself but not IL-6R to restrict the cytokine to form hexameric signaling complex and nullify inflammation due to IL-6 over expression.
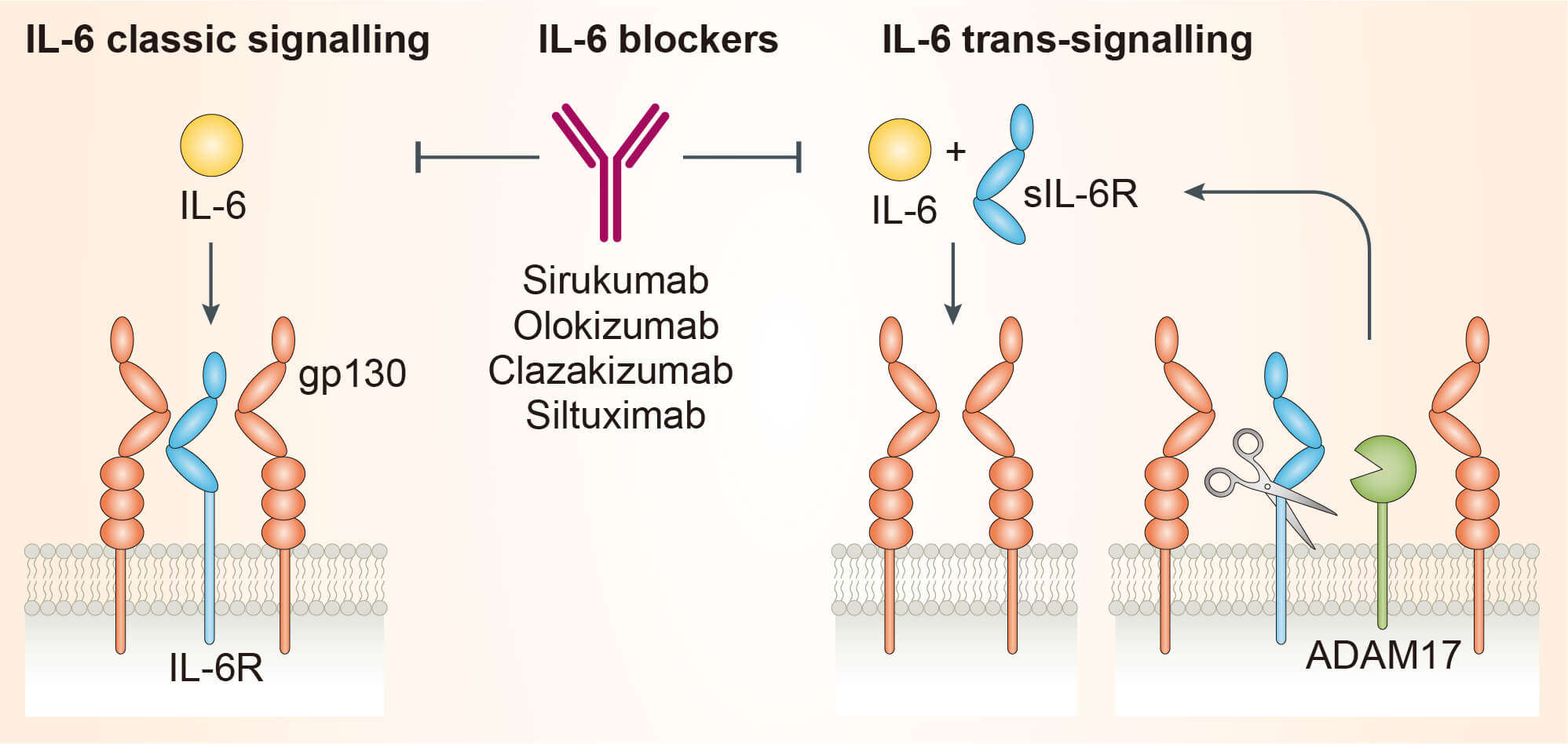 Fig.1 Mechanism of action of olokizumab
Fig.1 Mechanism of action of olokizumab
Clinical Projects of Olokizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02760433 | Recruiting | Rheumatoid Arthritis | R-Pharm | May 3, 2016 |
| NCT02760368 | Recruiting | Rheumatoid Arthritis | R-Pharm | May 3, 2016 |
| NCT02760407 | Recruiting | Rheumatoid Arthritis | R-Pharm | May 3, 2016 |
| NCT03120949 | Recruiting | Rheumatoid Arthritis | R-Pharm | April 19, 2017 |
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Olokizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

