Ovarian cancer is often diagnosed in advanced stages, and its resistance to existing treatment methods poses a significant challenge. According to data from the National Institutes of Health (NIH) in the United States, the five-year survival rate for diagnosed ovarian cancer is approximately 50%. Ovarian cancer shows low response rates to standard treatments such as chemotherapy, PARP inhibitors, and widely used immune checkpoint inhibitors.
In a recent study, researchers led by David B. Weiner from the Wistar Institute in the United States designed novel monoclonal antibodies (mAbs) that activate the immune system against cancer by binding to a unique surface receptor, attracting natural killer (NK) cells. They demonstrated the feasibility of using these antibodies alone or in combination with immune checkpoint inhibitors for the preclinical treatment of various types of ovarian cancer, including resistant and refractory ovarian cancer. The research findings were published on November 1, 2023, in the journal Science Advances, titled “Siglec-7 glyco-immune binding mAbs or NK cell engager biologics induce potent antitumor immunity against ovarian cancers.”
This new research, initiated through collaboration between Dr. Weiner and Dr. Mohamed Abdel-Mohsen at the Wistar Institute, explores the development of novel glycan signaling tools crucial for combating cancer.
In a small fraction of ovarian cancer patients who initially respond to existing therapies, treatment resistance becomes a concern over time, leading to tumor escape and cancer progression. Genetic mutations, such as the well-known BRCA gene mutations, significantly increase the risk of progressive ovarian cancer in women. The Centers for Disease Control and Prevention (CDC) estimates that over ten thousand women in the United States will die from ovarian cancer this year alone.
To counter treatment resistance in ovarian cancer, the researchers hypothesized that they could not only mobilize T cells activated by immune checkpoint inhibitors, such as PD-1 inhibitors, but also implement a strategy to activate NK cells through a conservative glyco-immune marker called Siglec-7 found on the surface of most NK cells.
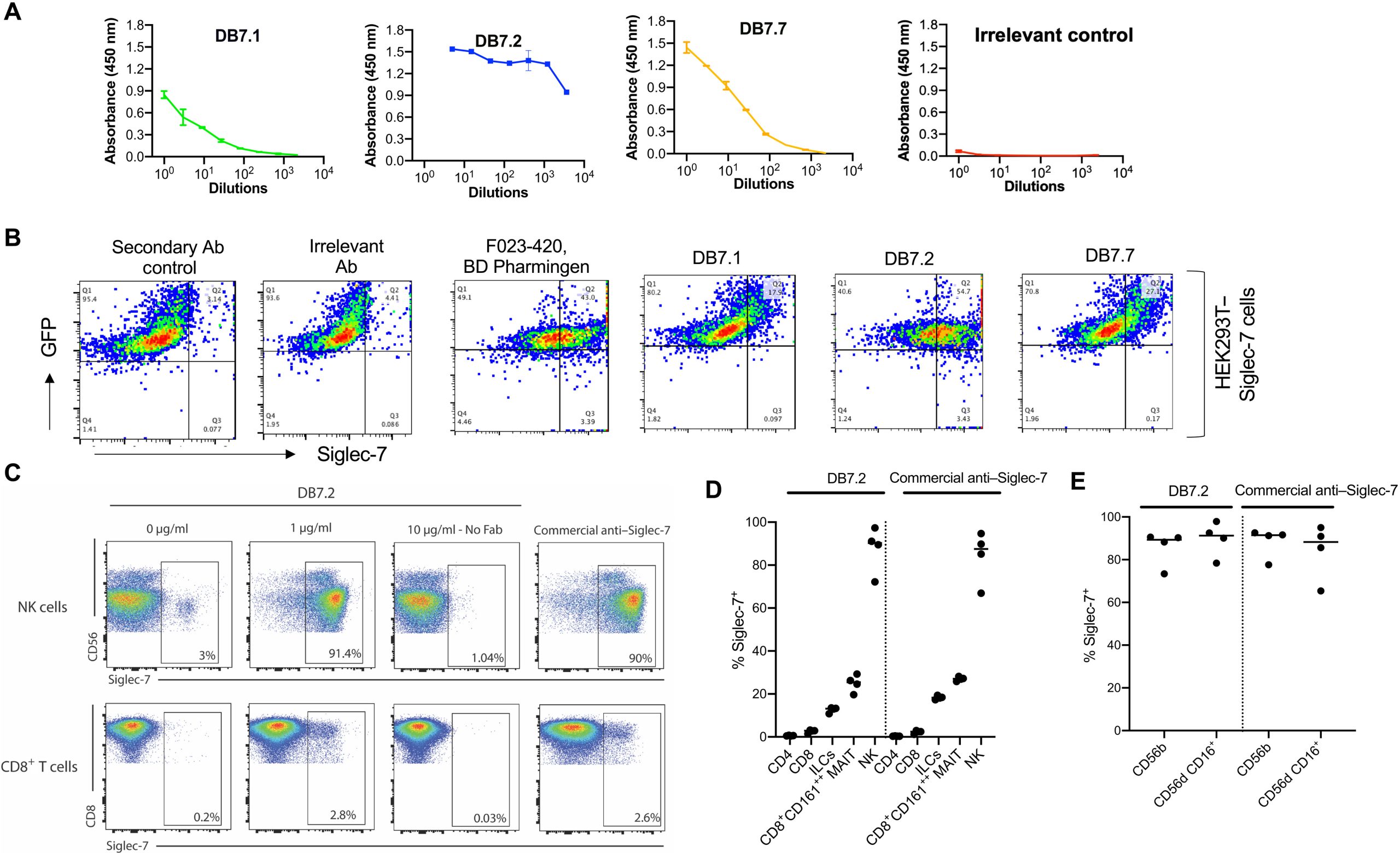
Recent studies have identified Siglec-7 expression in NK cells, prompting the researchers to test two new strategies targeting Siglec-7 to attract and activate NK cells against ovarian cancer.
The first approach used human mAbs discovered and developed by the Wistar Institute and a novel detection method to visualize and confirm that certain anti-Siglec-7 mAbs can activate human NK cells. In the presence of these antibodies, these activated NK cells responded to various human ovarian cancer cell lines. These activated NK cells killed ovarian cancer cells but did not harm non-cancerous cells treated with anti-Siglec-7 mAbs.
The researchers confirmed that anti-Siglec-7 mAbs could target various ovarian cancers carrying gene mutations such as BRCA1 and BRCA2. They further studied ovarian cancer treatment in humanized mouse models, observing that anti-Siglec-7 mAbs could slow down ovarian cancer growth, thereby improving survival rates.
After demonstrating the feasibility of using anti-Siglec-7 mAbs in an ovarian cancer model, the researchers believed there were other ways to further target ovarian cancer using these antibodies.
They hypothesized that by directly fusing the Siglec-7 activity binding site of anti-Siglec-7 mAbs with a second mAb previously developed to specifically bind to late-stage ovarian cancer unique to a molecule called follicle-stimulating hormone receptor (FSHR), a targeted anti-Siglec-7 bispecific antibody could be created. This antibody would be capable of binding to two different targets, forming a new class of NK cell engagers. This represents the second novel strategy against ovarian cancer.
The authors attempted to test whether this Siglec-7 NK cell engager could be effective by directly connecting potential cytotoxic NK cells with a “guided missile” specifically targeting ovarian cancer. In laboratory and humanized mouse challenge studies, Siglec-7 NK cell engagers effectively targeted ovarian cancer, activating nearby NK cells and efficiently killing various ovarian cancers.
In additional preliminary experiments, the authors found that Siglec-7 NK cell engagers could complement PD-1 immune checkpoint inhibitors. This is an important area that requires further investigation, and additional research may reveal more details about related mechanisms and potentially expand the application of PD-1 immune checkpoint inhibitors in ovarian cancer and other cancers.
Both Siglec-7 targeting approaches (anti-Siglec-7 mAbs and Siglec-7 NK cell engagers) recruited and activated populations of NK cells, reducing tumor size and extending survival time in animal models. The observed specificity of these two methods suggests that the vulnerability of Siglec in ovarian cancer can be exploited in treatment, perhaps with limited toxicity—a promising sign for future Siglec-related cancer research. However, the authors emphasize the importance of conducting more research in this area.
Dr. Weiner stated, “These findings open the door for us to further explore how to design Siglec-7 immunotherapies and other related molecules for the treatment of ovarian cancer and more resistant cancers. Further research may turn these approaches into new tools in our anti-tumor arsenal.”
As always, in the long journey from the laboratory bench to clinical applications, more research is needed to refine these techniques. However, this new study provides a different avenue for attempting to harness the unique interactions of Siglec-7 and other immune surface molecules for cancer treatment.
Dr. Devivasha Bordoloi, the first author of the paper, said, “We observed two methods targeting NK cells to control ovarian cancer in both dish and in vivo models. The prospects for this new research are vast, and I am excited to advance our studies to the next step.”
Reference
1. Bordoloi, Devivasha, et al. “Siglec-7 glyco-immune binding mAbs or NK cell engager biologics induce potent antitumor immunity against ovarian cancers.” Science Advances 9.44 (2023): eadh4379.