Osteosarcoma (OS) is the most common malignant bone tumor, primarily affecting adolescents. In the 1970s, the clinical use of chemotherapy in conjunction with surgical resection significantly improved the five-year survival rate of OS patients. However, since then, there have been limited treatment advancements in further improving clinical outcomes for OS patients.
Whole-genome and exome sequencing of OS have unveiled the genomic landscape of OS and highlighted the prevalence of chromothripsis, a phenomenon where multiple chromosomal rearrangements occur simultaneously in certain genomic regions, leading to extensive genomic mutations in OS patients.
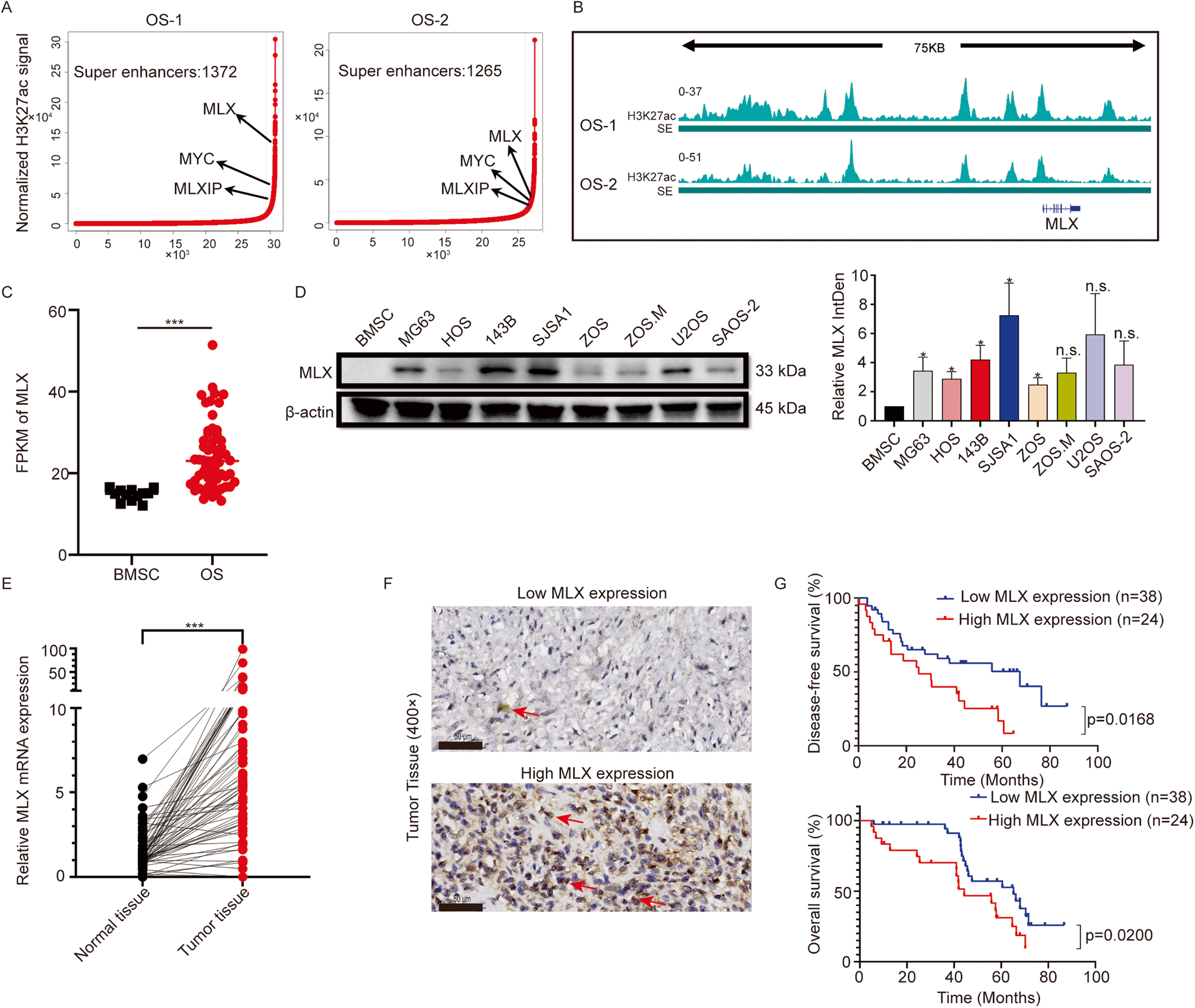
Recently, researchers from the First Affiliated Hospital of Sun Yat-sen University published an article titled “Super-enhancer-driven MLX mediates redox balance maintenance via SLC7A11 in osteosarcoma” in the journal Cell Death and Disease. This study reveals that the super-enhancer-driven MLX maintains the oxidative-reductive balance in osteosarcoma through targeting SLC7A11.
Osteosarcoma is a common bone tumor with limited treatment progress over the past three decades. The prevalence of transcription addiction in cancer cells underscores the biological significance and clinical relevance of super-enhancers. In this study, researchers discovered that a member of the Myc-MLX network, Max-like Protein X (MLX), is driven by super-enhancers.
Upregulated MLX expression is associated with poor prognosis in osteosarcoma. Knocking out the MLX gene affects osteosarcoma growth and metastasis. Transcriptome sequencing indicates that MLX is involved in various metabolic pathways, such as lipid metabolism, and can induce metabolic reprogramming. Furthermore, MLX gene knockout disrupts iron transport and storage, leading to increased intracellular iron levels and subsequently triggering ferroptosis.
Mechanistically, MLX regulates the glutamate/cystine antiporter SLC7A11, promoting the uptake of extracellular cystine, which is essential for the synthesis of the antioxidant glutathione (GSH). This detoxifies reactive oxygen species (ROS) and maintains the oxidative-reductive balance in osteosarcoma cells. Importantly, the FDA-approved anti-inflammatory drug sulfasalazine can inhibit SLC7A11, disrupt the redox balance, and induce extensive ferroptosis, inhibiting tumor growth in vivo.
In summary, this study identifies MLX as a member of the extended MYC family driven by super-enhancers. High MLX expression promotes cystine uptake through SLC7A11, facilitating GSH biosynthesis to detoxify ROS and maintain the oxidative-reductive balance. Disrupting MLX-SLC7A11 impairs cystine uptake, leading to ROS accumulation and subsequent lipid peroxidation, ultimately triggering ferroptosis. Sulfasalazine, an FDA-approved drug, can induce ferroptosis in PDX models, highlighting its clinical potential in the treatment of osteosarcoma.
Reference
1. Guo, Weitang, et al. “Super-enhancer-driven MLX mediates redox balance maintenance via SLC7A11 in osteosarcoma.” Cell Death & Disease 14.7 (2023): 439.