The latest update—
Cases of COVID-19 in the U.S. at a Glance on April 3, 2020, which include both confirmed and presumptive positive cases of COVID-19 reported to CDC or tested at CDC since January 21, 2020
- Total cases: 213,144
- Total deaths: 4,513
Lately there has been an inspiring advance in the therapeutics for COVID-19 that a modified mRNA vaccine from Moderna Therapeutics started phase 1 clinical testing, which spent just 42 days to produce the first batches of the vaccine mRNA-1273. The vaccine encodes a prefusion-stabilized form of the 2019-nCoV (SARS-CoV-2) spike (S) protein, which brings hope mingled with concerns to scientists that its earliest time of clinical stage would be 2021, too late in the day to deal with the current pandemic, let along the numerous technical, capital or policy challenges that may occur during its clinical development and manufacture.
Facing the challenge, pharmaceutical companies are devotedly developing other therapeutic molecules through discovery and development pipelines, some of which are approved and already used as adjunct therapies, including Fujifilm Toyama Chemical’s favipiravir and Gilead’s remesdivir. Other useful approaches can be monoclonal antibodies (mAbs) targeting the previous coronaviruses like severe acute respiratory syndrome (SARS) virus and Middle Eastern respiratory syndrome (MERS) virus, small interfering RNAS (siRNAs), DNA vaccines, etc., but we all know that the real headache lies in the clinical development stages, which is too long to defeat current ‘epidemic enemy’.
Currently some measurements are taken with the aim to reverse the present situation and to deal with the next possible health crisis in advance.
- Focused repurposing of existing drugs
Focused repurposing of approved small-molecule compounds seems to be the most promising way to develop computational drugs targeting SARS-CoV-2 based upon the 3CL protease from the SARS virus, but there are technical limitations on the AI systems or other in silico approaches. In addition, the development process may not come to the result inspection stage when there is a lack of continued funding, or ending of the epidemic, just like the single phase 1 drug study (NCT00215826) and two phase 1 vaccine studies targeting SARS, in 2003.
- Preparation for therapeutic mAb targeting specific pathogens in advance
Researchers believe that, non-market mechanisms like the Platform for European Preparedness Against Re-Emerging Epidemics (PREPARE) out of Antwerp, Belgium, and the US Defense Advanced Research Projects Agency (DARPA)’s Pandemic Prevention Platform (P3) program can help in that they take action in advance, of which the latter has paid attention to some mAb programs for COVID-19 that can cut down preclinical duration.
In-process Monoclonal Antibodies (mAbs) for COVID-19
| Company | Modality |
| AbCellera Biologics | Fully human IgG1 mAbs targeting SARS-CoV-2 |
| EUSA Pharma | Sylvant (siltuximab), human IgG1κ mAb against IL-6 |
| ImmunoPrecise Antibodies | Fully human IgG1 mAbs targeting multiple undisclosed epitopes on SARS-CoV-2 |
| InflaRx | Fully human IgG1 mAb against complement factor 5a |
| Vir Biotechnology | Human IgG1 mAbs targeting SARS-CoV-2 |
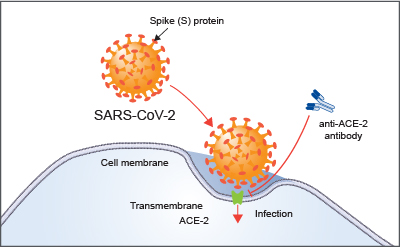
Mechanism of SARS-CoV-2 infecting human body determines that antibodies against specific proteins on the surface of SARS-CoV-2 are an intuitive approach.
The process of SARS-CoV-2 infecting normal cells can be roughly divided into three steps: invasion, genome replication and transcription, production of virus proteins and assembly of new viruses. Different proteins play varied roles in this process. For example, the spike glycoprotein (s protein) on the surface of the virus is the key for the virus to invade the host cell. Without the binding of S protein to the receptor molecules on the surface of host cells, the viral envelope could not fuse with the host cell membrane, and could not cause infection. When SARS-CoV-2 invades human body, its S protein first specifically binds to the receptor angiotensin-converting enzyme 2 (ACE2), which then translates the RNA polymerase, transcribing its own genes into mRNA and constructing other structural proteins.
Alternative mAbs that are applicable for research
Other therapeutic rabbit/mouse/humanized monoclonal antibodies of various crucial target spots of SARS-C0V-2 can join to improve the odds, including:
- 2019-nCoV S receptor binding domain (RBD, responsible for recognizing the cell surface receptor.)
- 2019-nCoV NP (N protein, required for coronavirus RNA synthesis, has RNA chaperone activity that may be involved in template switch.)
- 2019-nCoV S1 & S2 (S1 consists a receptor binding domain; S2 composes elements required for the membrane fusion.)
Joint efforts should be made between pharmaceuticals and antibody engineers
On 12 March 2020, Vir announced that Biogen committed to being its North American development and clinical manufacturing partner for the proprietary anti-SARS-CoV-2 mAbs. There is no doubt that only with the joint efforts of the government, pharmaceutical companies, and antibody manufacturers, could the clinic process be expedite, and effective drugs & vaccines enter the clinic test as soon as possible.
Reference
1. Simonian M, “Covid-19 Vaccines, Immunity, And Effect On Children.” Medicine and Healthcare Reports 2.4 (2021).