In a recently published study, researchers from the Max Planck Institute for Dynamics and Self-Organization in Germany, the University of Cologne, and the University of Washington found that a carefully designed mixture of broadly neutralizing antibodies (bNAb) may be able to help treat HIV infection while minimizing the risk of the virus evading treatment. The findings were published online July 19, 2022, in the journal eLife titled “Design of an optimal combination therapy with broadly neutralizing antibodies to suppress HIV -1”.
This study demonstrates that a computational approach to selecting bNAb combinations based on viral genetics aid in preventing viral escape and improving HIV treatment efficacy. It may also provide a strategy for designing effective combinations of bNAs to treat other rapidly evolving pathogens.
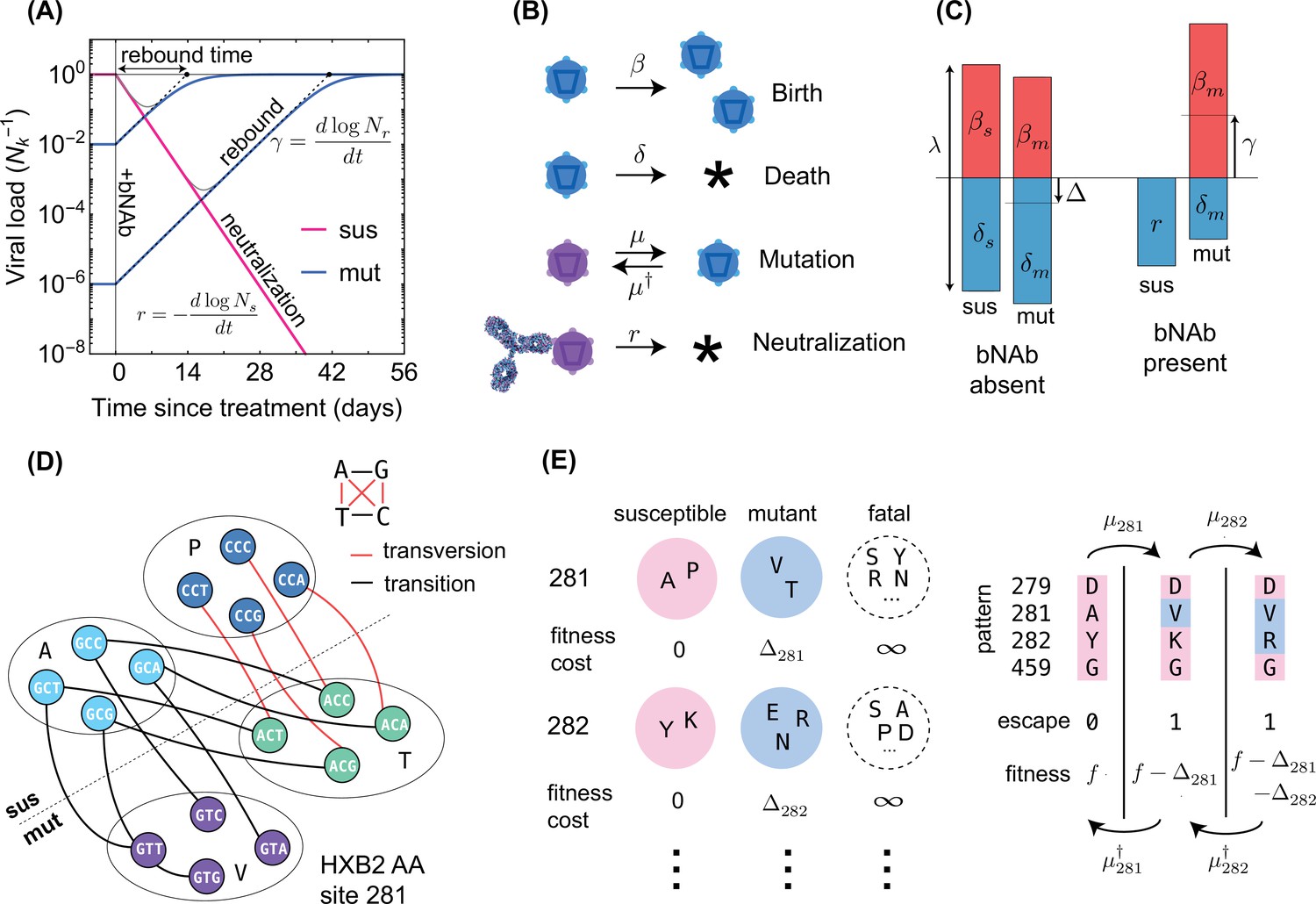
bNAb represents a promising new tool for the treatment or possible treatment of rapidly evolving viral infections such as HIV. Clinical trials using a single bNAb to treat HIV infection revealed that some HIV strains may evade treatment and trigger viral rebound in the blood. Therefore, using bNAb combinations may be a more effective approach, but determining the best combination is a challenge.
Colin LaMont, the first author of the paper and a researcher at the Max Planck Institute for Dynamics and Self-Organization, said, “In our study, we propose to use a computational approach to predict the effectiveness of HIV genetics-based bNAb combinations.”
LaMont and colleagues analyzed genetic signatures of HIV collected from 11 untreated patients over a 10-year period using high-throughput sequencing technologies. They utilized the information to predict which HIV strains might be able to evade treatment with different bNAb and whether evasion of bNAb treatment was associated with survival costs. Following that, they employed computational approaches to forcasting viral rebound in three real bNAb clinical studies.
Finally, these authors discovered the combination of bNAb that was least likely to allow any viral evasion. It was also discovered that some bNAb (e.g., 10-1074) were better at fighting different virus populations because the mutations allowing for viral escape also made such viruses less likely to survive. Other bNAb (including PGT121) were more effective against less diverse virus populations because mutations that allow viruses to escape are rare. It was finally suggested in these findings that the optimal bNAb combination are PG9, PGT151, and VRC01.
LaMont said, “We found that the combined use of PG9, PGT151, and VRC01 lowered the chance of viral rebound to less than 1%. This was accomplished by targeting three distinct regions of the protective outer envelope of the virus.”
According to Armita Nourmohammad, corresponding author of the paper and an assistant professor in the Department of Physics at the University of Washington, “Combining bNAb, which is given by intravenous injection every few months, with antiretroviral therapy (ART), which currently requires daily dosing, may further improve long-term treatment efficacy against HIV.”
ART reduces the ability of HIV to proliferate and generate new variants, limiting the genetic diversity of the HIV viral population and reducing the possibility of developing bNAb escape variants. More studies are needed to validate the potential benefits of combining ART and bNAb, according to the authors.
“Our study suggests that the use of genetic data may help design more effective therapies against HIV, and even other rapidly evolving pathogenic factors, such as hepatitis C virus (HCV), drug-resistant bacteria, and cancerous tumor cells.” Nourmohammad Concluded.
Reference
1. LaMont, Colin, et al. “Design of an optimal combination therapy with broadly neutralizing antibodies to suppress HIV-1.” Elife 11 (2022): e76004.