Some HIV-1 carriers who received early antiretroviral therapy (ART) for several years can maintain long-term control over the virus after treatment interruption. However, the mechanism behind this post-treatment control is not fully understood.
In a new study, researchers from the Pasteur Institute in France, the French National Institute of Health and Medical Research (Institut National de la Santé et de la Recherche Médicale), and the Paris Public Hospital Network, for the first time, investigated and revealed how neutralizing antibodies, including broadly neutralizing antibodies (bNAbs), contribute to this virus control. The research results were published online in the journal “Cell Host & Microbe” on July 10, 2023, under the title “Anti-V1/V3-glycan broadly HIV-1 neutralizing antibodies in a post-treatment controller.”
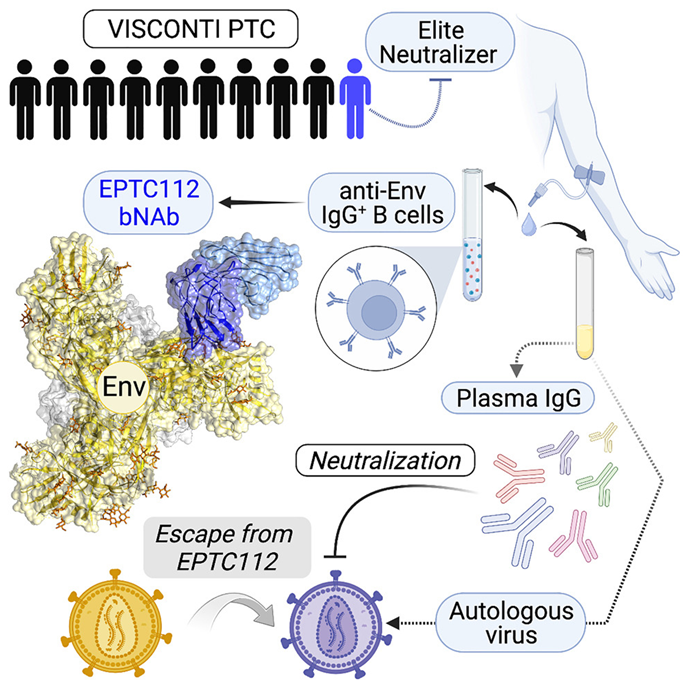
The term “post-treatment controller” is used to describe rare HIV-1 carriers who started treatment early and maintained it for several years, being able to control the virus for years after treatment cessation. These individuals were identified several years ago through the VISCONTI study, which brought together the largest cohort of long-term post-treatment controllers in France.
Although the mechanism of achieving long-term remission of HIV-1 infection in the absence of ART treatment is not yet fully understood, identifying these cases provides a unique opportunity to enhance scientists’ understanding of factors related to HIV-1 infection control.
A study conducted by a team led by Dr. Hugo Mouquet from the Pasteur Institute’s Humoral Immunology Group, in collaboration with Dr. Asier Sáez-Cirión, head of the Virus and Immunity Control Group at the Pasteur Institute’s Virus and Immunity Control Group, is now contributing to a more detailed description of these mechanisms. Asier Sáez-Cirión explained, “Our research in 2020 on the immune responses of post-treatment controllers marked an important first step in confirming that some of these individuals have a potent and effective antibody response against HIV-1, which may contribute to virus control.”
Now, this new study further advances this understanding. By examining the role of antibodies in specific “post-treatment controller” cases with particularly high levels of broadly neutralizing antibodies in their serum, these authors found that disease remission might be associated with the activity of these antibodies.
Hugo Mouquet described this finding: “Our study is the first to describe broadly neutralizing antibodies (bNAbs) targeting HIV-1 envelope proteins in post-treatment controllers, with the antibody EPTC112 being one of the most active members of this family.” EPTC112 antibodies can neutralize about one-third of the HIV-1 virus variants tested in vitro and can induce the elimination of infected cells in the presence of natural killer (NK) cells, immune cells that eliminate abnormal cells in the body.
Therefore, this new research provides critical information on how broadly neutralizing antibodies like EPTC112 may change the course of HIV-1 infection within patients from the VISCONTI cohort. While the virus circulating in this patient’s body developed resistance to EPTC112 due to mutations in the targeted region, other antibody populations isolated from the patient’s blood could effectively neutralize the virus. Thus, the study suggests that broadly neutralizing antibodies from the EPTC112 family exert selective pressure on the HIV-1 virus.
Although the virus escaped the action of these bNAbs, it remains susceptible to neutralization by other anti-HIV-1 antibodies produced in the patient’s body. This observation suggests cooperation between multiple populations of neutralizing antibodies.
Hugo Mouquet explained, “We have identified a potential link between the production of neutralizing antibodies, including bNAbs, and the control of HIV-1. This is exciting for a better understanding of the underlying mechanisms of virus control, especially through studying more post-treatment controllers with similar characteristics. Indeed, we hope to continue studying the antibody responses of other ‘post-treatment’ controllers in the near future to see if they also contribute to long-term remission of the infection.”
This discovery opens up new avenues for HIV-1 treatment and offers hope for achieving remission without the use of ART through the use of bNAbs. France is set to begin clinical trials of bNAbs by the end of 2023.
“This Phase II trial, conducted through the ANRS RHIVIERA consortium, in collaboration with the Pasteur Institute, the Paris Public Hospital Network, the French National Institute of Health and Medical Research, and the Rockefeller University in New York, will investigate combining short-term ART treatment with two different bNAbs targeting different regions of the HIV-1 envelope protein during the primary infection stage, compared to a placebo. After a year of close monitoring, and according to a set of detailed criteria, it may be possible to stop treatment. This trial will allow us to determine whether this treatment strategy can induce sufficient immune responses to control the infection after stopping ART treatment,” summarized Hugo Mouquet.
Reference
1. Molinos-Albert, Luis M., et al. “Anti-V1/V3-glycan broadly HIV-1 neutralizing antibodies in a post-treatment controller.” Cell Host & Microbe 31.8 (2023): 1275-1287.