Cellular secretions play various crucial roles within the body. However, methods to connect this functional information with surface markers and the transcriptome have been lacking. In a recent research paper published in the international journal “Nature Communications,” titled “SEC-seq: Association of Molecular Signatures with Antibody Secretion in Thousands of Single Human Plasma Cells,” scientists from institutions including the University of California, Los Angeles, have gained new insights into the genes responsible for producing and releasing immunoglobulin G (IgG), the most common antibody type in the human body. This research holds the potential to advance the development of novel therapies based on antibodies for human diseases such as cancer and arthritis, as well as medical treatments relying on antibody production.
Antibodies are a category of proteins vital to the body’s immune system. Immunoglobulin G (IgG) can store memories of past infections and mark dangerous microorganisms for destruction by immune cells. Maternal IgG is crucial for the immune defense of newborns. Decades ago, researchers knew that white blood cells known as plasma B cells could produce IgG efficiently. These cells are capable of producing over ten thousand IgG molecules per second. However, the molecular mechanisms behind promoting antibody secretion into the bloodstream have remained unclear.
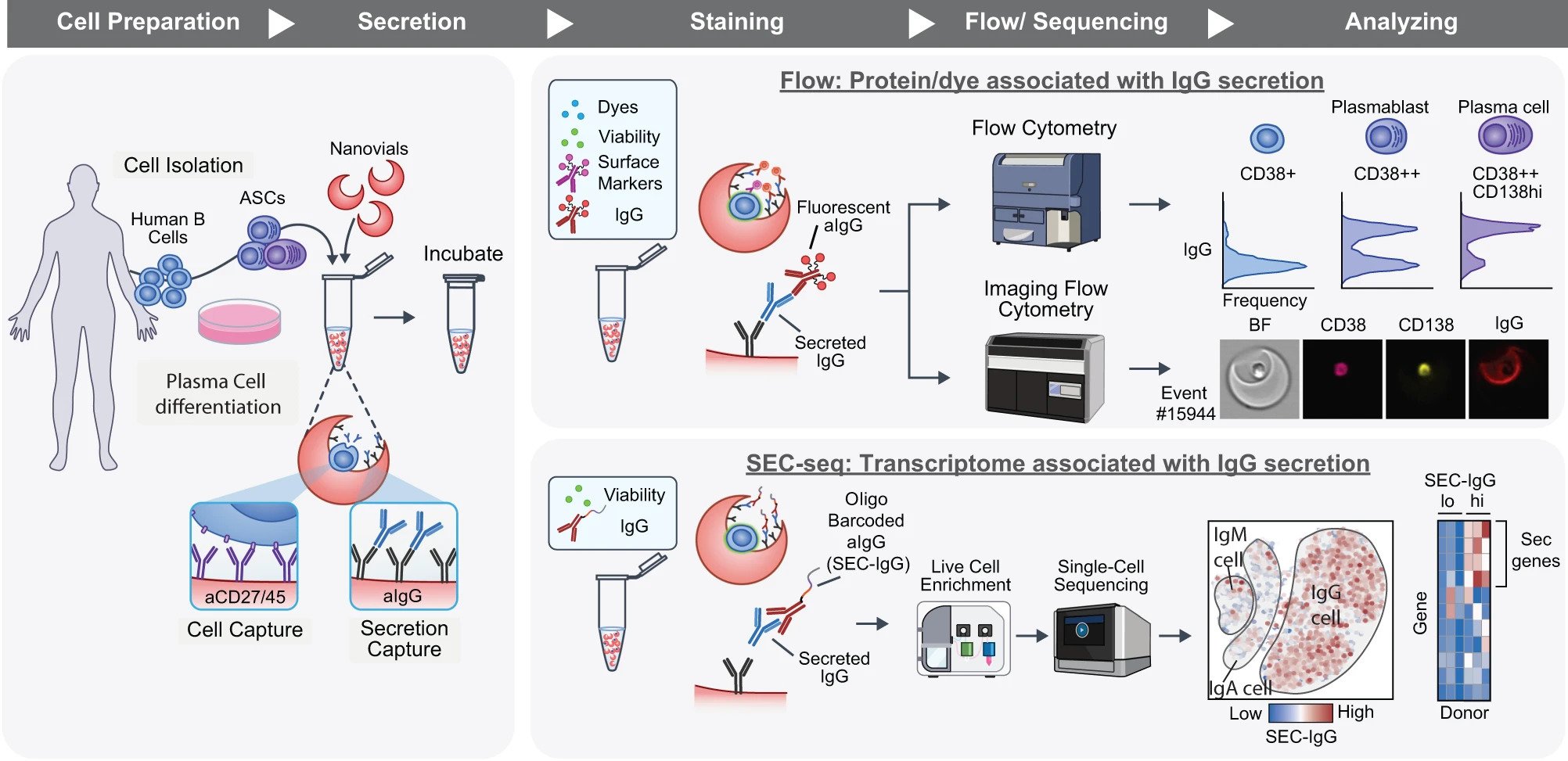
To gain further insights into these mechanisms, researchers conducted an unprecedented analysis. They captured thousands of individual plasma B cells and their secretions, then correlated the amount of protein released by each cell with the expression patterns of tens of thousands of genes in that cell. To collect cells and their secretions, researchers used nanovials, tiny bowl-shaped hydrogel containers. The analysis results indicated that genes involved in producing energy and eliminating abnormal proteins were more critical for the secretion of a large amount of IgG compared to genes responsible for instructing antibody production itself. Additionally, they discovered that the presence of the CD59 gene, previously unrelated to IgG secretion, was a better predictor of high-producing plasma cells than other genetic markers associated with these cell types.
Dino Di Carlo, one of the researchers, likened these cellular processes to a protein manufacturing assembly line with bottlenecks occurring in many places. Everything inside the cell must proceed smoothly in sync. When a cell is producing a large quantity of protein, it consumes a substantial amount of energy. Therefore, there is a need for a method to correct protein misfolding that may occur. These research findings not only advance our fundamental understanding of biology but also hold clinical significance in the field of biomedical research. For example, knowing which genes are associated with high antibody production could enable pharmaceutical companies to modify cells to secrete more antibodies. This knowledge also has the potential to be applied in emerging strategies that directly introduce engineered cells into patients’ bodies.
In this study, the novel methods involving nanovials and standard laboratory equipment used by the researchers offer possibilities for understanding how DNA instructions translate into cellular behavior. Each nanovial contains specific molecules that can customarily bind to proteins on the cell surface under investigation. This allows the nanovials to capture one cell at a time. Once the cells are trapped and protected inside the nanobowls, their secretions accumulate and adhere to specific antibodies designed to capture them. In this research, researchers “trapped” thousands of plasma cells along with their IgG secretions in nanovials. These nanovials have a diameter only about one-third the thickness of a sheet of paper. Subsequently, the researchers analyzed the mRNA of each cell using instruments. Every cell in the human body carries the same blueprint written by DNA. Therefore, researchers can detect which genes are active by observing mRNA, which translates these instructions so that each cell can produce proteins matching its function. Carlo, one of the researchers, noted that each cell contains multiple layers of information, and they can now connect the final layer (the actual amount of protein secretion with a clear function in the human body) with the most fundamental layer of genetic code. Currently, there is no technology capable of achieving this, and the most intriguing question for researchers is what questions to ask next. In future research, researchers hope to identify all the genes influencing plasma cell production and IgG secretion.
In summary, the new method developed by researchers links secretion quantity with single-cell sequencing (SEC-seq), allowing researchers to delve deeper into the connection between the genome and function. This lays a foundation for discoveries in fields such as immunology and stem cell biology.
Reference
1. Cheng, Rene Yu-Hong, et al. “SEC-seq: Association of molecular signatures with antibody secretion in thousands of single human plasma cells.” Nature Communications 14.1 (2023): 3567.