Neoantigens are peptides derived from nonsynonymous mutations in human leukocyte antigens (HLAs) that are recognized by antitumor T cells. The large diversity of HLA alleles and limited clinical samples often narrow scientists’ capacity to investigate the blueprint for the landscape of neoantigen-targeted T-cell responses in patients during therapy. In a recent study published in the journal Nature titled “Neoantigen-targeted CD8+ T-cell responses with PD-1 blockade therapy,” scientists from the University of California and other institutions have identified and analyzed for the first time the steps by which immune cells “see” and respond to cancer cells. The findings may help shed light on why some therapies work for certain cancer patients but not others.
The researchers indicate that the findings may hold promise for helping with the development of better and more personalized immunotherapies, even for patients whose immune systems do not currently seem to be able to respond to therapies.
“This is an important step forward in our understanding of what T-cell responses can be observed in tumors and how they change over time when they are in the tumor and blood circulation to find new tumor cells to attack,” said Dr. Cristina Puig-Saus. “Greater knowledge of how the T cell response clears metastatic tumor masses may help us design better drugs as well as manipulate T cells diversely to imitate them.”
The researchers employed sophisticated gene editing techniques to examine the immune responses of metastatic melanoma patient organisms receiving anti-PD-1 checkpoint inhibitor treatment for the first time. Although T cells identify and eliminate mutations in cancer cells, leaving normal cells unharmed, cancer cells typically evade the host organism’s immune system, and checkpoint inhibitors can be created to boost T cells’ capacity to recognize and target cancer cells. Based on the results of this study, researchers will be able to determine precisely what a particular patient’s immune system can identify in their cancer to effectively distinguish it from normal cells and target the cancer cells.
The researchers commented that when immunotherapy is effective, it directs a diverse set of T cells to defend against a small subset of selected mutations in the tumor, and these T cell responses expand and develop in the tumor and bloodstream over the course of the therapy. The results of this study indicate that patients who do not respond to therapy still induce a tumor-responsive T-cell response, that these T cells can potentially be isolated and that their immune receptors can be used to genetically modify larger numbers of T cells to regain tumor resistance in the patient’s body, and that these T cells can be expanded in culture and reinfused into the patient’s body, according to Dr. Cristina Puig-Saus.
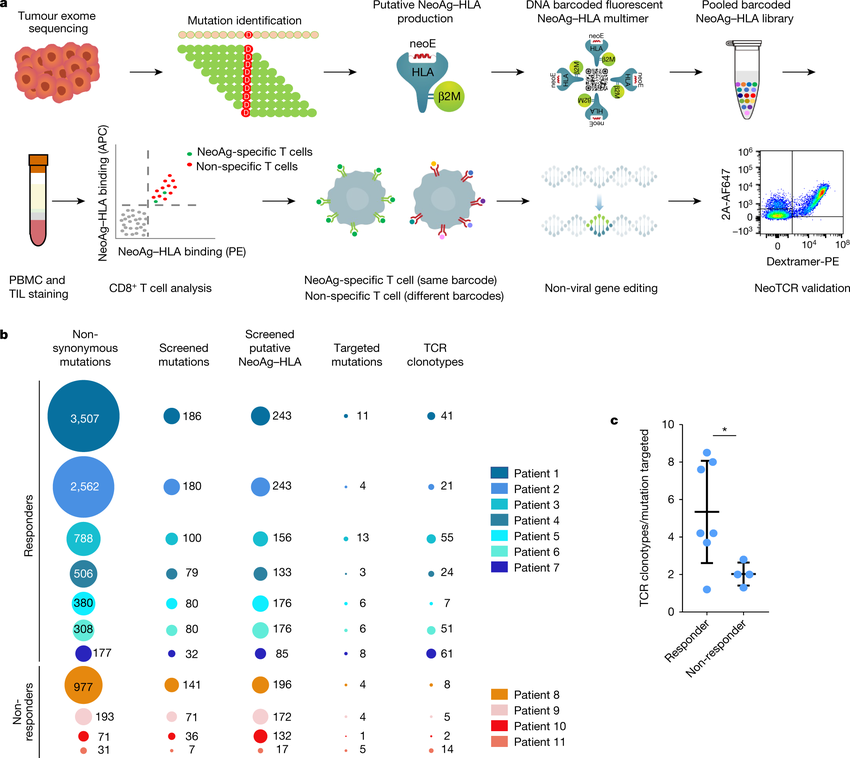
Of the 11 patients studied, seven responded to PD-1 blockade, while four did not. The number of mutations in the tumors ranged from 3507 to 31, but despite this wide range, the number of mutations observed in tumor-reactive T cells ranged from 13 to 1. In one of the patients who had a clinical benefit to the therapy, the response of the patient’s organism was diverse, with different mutation-specific T cells isolated from blood and tumor, ranging from 61 to 7. Patients who lacked a response to the therapy, on the other hand, had 14–2 T cell types identified.
In addition, in patients who responded to therapy, researchers were able to isolate tumor-responsive T cells from the patients’ blood and tumors throughout the course of treatment, but in patients who did not respond, T cells were not repeatedly detected. However, this paper’s findings imply that immunological receptors from all patients’ T cells can shift the specificity of immune cells against the tumor, whether or not a response is established.
In summary, the results of this paper suggest that effective anti-PD-1 immunotherapy is often associated with the presence of polyclonal CD8+ T cells in tumors and blood, and that these cells are specific for a limited number of immunodominant mutations that are repeatedly identified over time.
Reference
1. Puig-Saus, Cristina, et al. “Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy.” Nature 615.7953 (2023): 697-704.