High-Throughput Antibody Production Services
From gene sequence to hundreds of purified recombinant antibodies in as little as two weeks. Empower your screening, characterization, and lead optimization with unparalleled speed, scalability, and reliability.
Why High-Throughput Antibody Production?
In modern antibody discovery and development, the ability to rapidly screen a large number of candidates is critical for success. Traditional antibody production methods, however, are often slow, labor-intensive, and inconsistent, creating a significant bottleneck that delays critical project milestones.
The High-Throughput Solution
We've engineered a streamlined, automated, and high-throughput (HT) platform specifically designed to solve this challenge. By leveraging modern biotechnology, automated equipment, and large-scale screening techniques, we enable the parallel production of dozens to thousands of recombinant antibodies. This approach saves valuable time, reduces costs, and enhances the success rate of your research and development.
Our platform handles the entire workflow—from gene synthesis to purified antibody—allowing your team to focus on downstream analysis and discovery. We utilize efficient mammalian cell expression systems to ensure the accurate folding and modification of your antibodies, providing the high-quality material you need for comprehensive screening and validation campaigns.
Why Choose Our High-Throughput Platform?
We integrate cutting-edge technology with automated processes to provide you with superior, efficient, and reliable antibody production services.
Massive Scalability
Whether you need 24 or over 1,000 unique antibodies, our platform is built to handle your project's scale with consistent results.
Exceptional Consistency
Our automated and highly controlled processes minimize batch-to-batch variability, ensuring your screening data is reliable.
Broad Diversity
By employing high-throughput screening, we access larger and more diverse antibody libraries, increasing the likelihood of discovering effective antibodies.
Enhanced Optimization
Our technology enables parallel optimization of antibody parameters like affinity and specificity, facilitating faster identification of optimal variants.
Format Flexibility
We produce a wide range of antibody formats, including IgG, IgM, IgE, Fab, scFv, and bispecific antibodies to meet your specific needs.
Robust Adaptability
Our platform seamlessly integrates with AI, phage display, and advanced data analysis, making your development process more efficient.
Streamlined Production Process
Experience our transparent, end-to-end workflow, engineered for maximum efficiency to take your project from gene synthesis to delivery in as little as two weeks.
Antibody Sequence
Gene Synthesis
Vector Construction
Transfection & Expression
Antibody Purification
Quality Control
Service Details & Specifications
Our High-Throughput Production platform is expertly designed to serve as the downstream engine for a variety of advanced antibody discovery methods, empowering you to rapidly transition from candidate identification to functional antibody production and screening.

| Parameter | Specification |
|---|---|
| Expression System | High-yield HEK293 or CHO-S transient expression systems |
| Throughput | Up to 1,000+ antibodies per project |
| Production Scale | From microgram (µg) to milligram (mg) scale. Standard tiers: 50µg, 100µg, 500µg, 1mg |
| Antibody Formats | IgG, IgM, IgE, Fab, scFv, BsAb, and more |
| Purity Guarantee | Standard: >95% (by SDS-PAGE). High Purity: >98% also available. |
| Turnaround Time | 2-4 weeks, depending on project scale and complexity |
Advanced Equipment & Technology
Our state-of-the-art facilities are equipped with cutting-edge instrumentation to ensure precision, scalability, and high-quality results for every project.
Case Studies
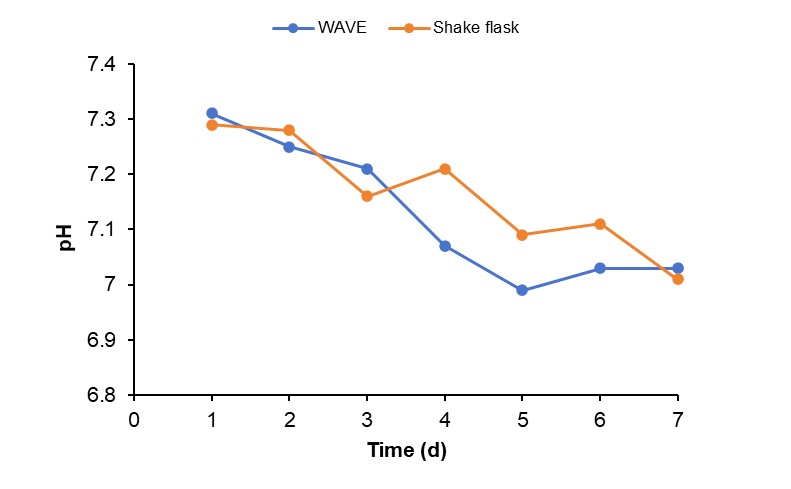
Scalable Production in WAVE® Bioreactor
Challenge: To establish a scalable and reliable process for producing recombinant antibodies in HEK293F cells that bridges the gap between lab-scale shaker flasks and larger production volumes, while maintaining comparable cell growth, viability, and antibody productivity.
Solution: We implemented the single-use WAVE® rocking bioreactor system. The process involved expanding HEK293F cells and inoculating them into a 25L Cellbag bioreactor at a starting density of 6.0x10⁵ cells/mL in 5L of medium. The culture was transfected after 24 hours and cultivated for 7 days, with key parameters monitored throughout.
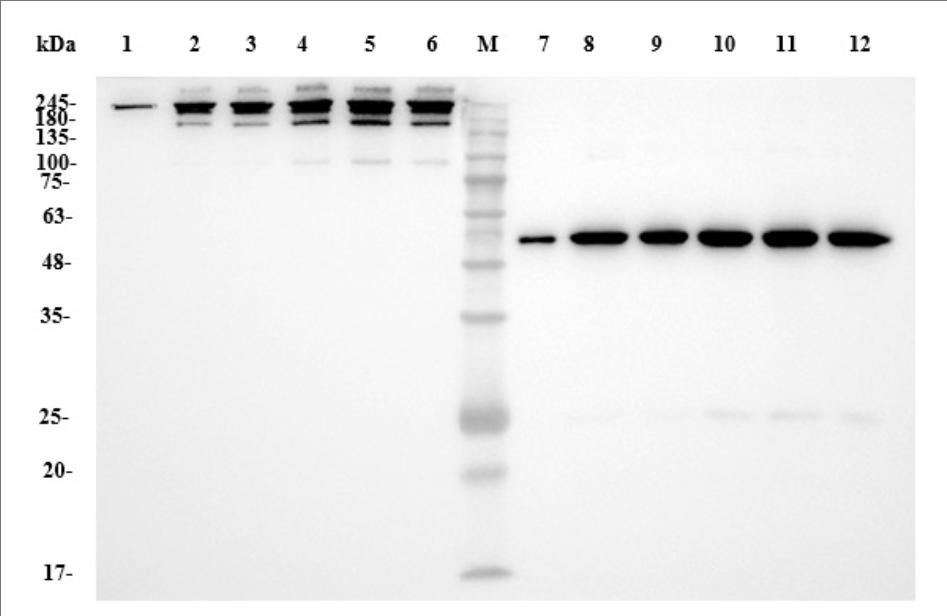
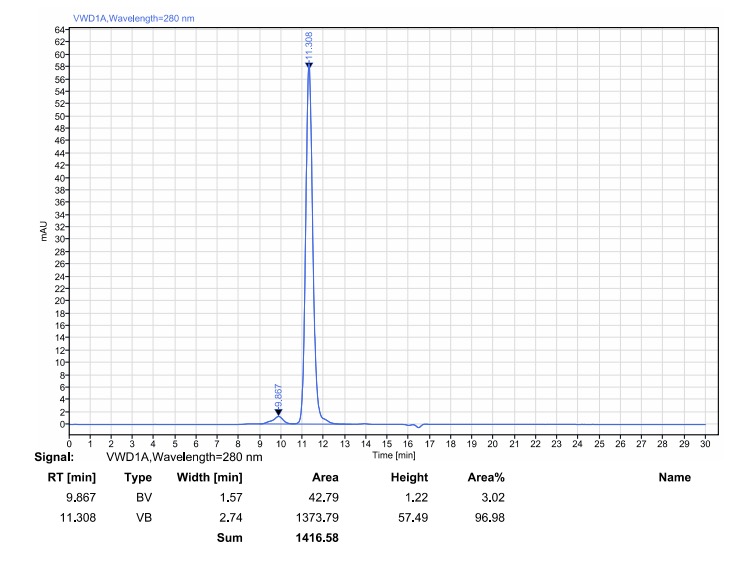
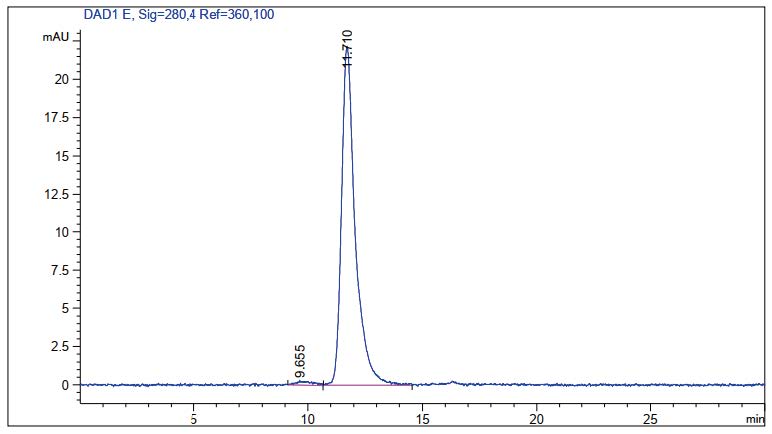
Results: The WAVE® bioreactor system demonstrated a robust and reproducible production process, with cell growth and viability profiles comparable to the shake flask control. The final purified antibody showed >95% purity by SDS-PAGE and SEC-HPLC analysis, confirming the system as a reliable and scalable solution for production up to 25L.
Download Full Case StudyProduction of a Research-Grade Biosimilar
Challenge: To produce and characterize a research-grade biosimilar anti-PD-1 antibody, ensuring it matches the originator in terms of purity, binding affinity, and biological function.
Solution: A comprehensive workflow was established, including gene synthesis, expression in our serum-free mammalian platform, and multi-step purification. Extensive characterization was performed using SDS-PAGE, SEC-HPLC, ELISA, Dot Blot, Western Blot, and Surface Plasmon Resonance (SPR).
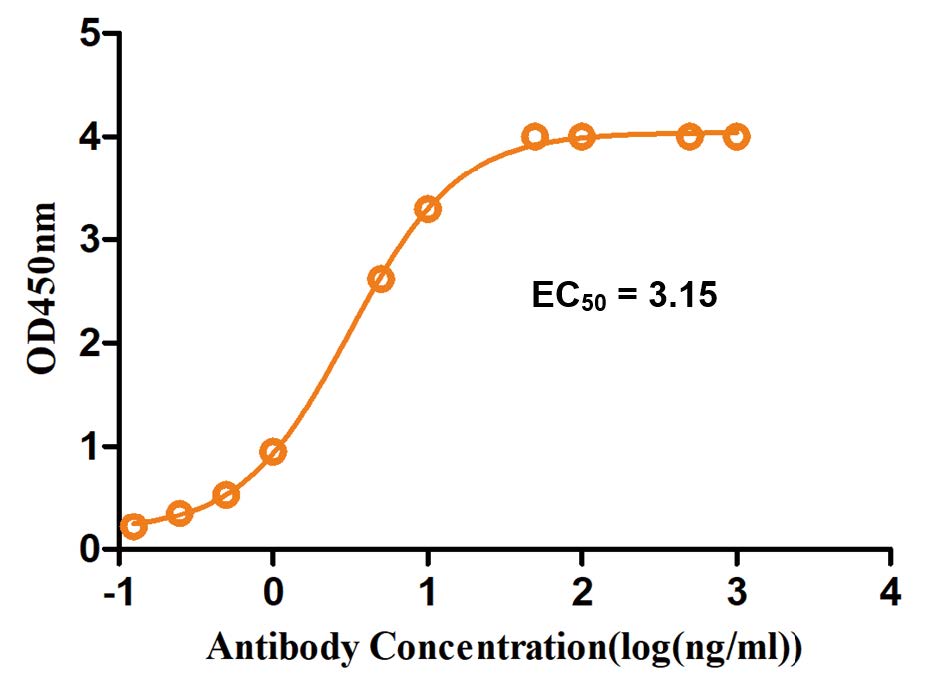
Results: The purified anti-PD-1 antibody demonstrated high purity and strong binding affinity to human PD-1 (KD of 1.09 nM). Functionally, the antibody effectively blocked the PD-1/PD-L1 interaction and significantly promoted IFN-γ secretion in a PBMC-based assay, confirming its potent bioactivity and validating the success of the project.
Download Full Case StudyOur Featured Antibody Products & Services
Explore our highlighted antibody products, meticulously developed for superior performance, or submit a request for your unique custom antibody production needs today.
Featured Products
- Humanized Antibodies
- Chimeric Antibodies
- Mouse Antibodies
- Fab Fragment Antibodies
- ScFv Antibodies
- IgM Antibodies
Need Custom Production?
Beyond our extensive catalog of ready-to-use antibodies, we excel at providing fully customized production services meticulously tailored to your unique research objectives. Our expert team is equipped to handle a comprehensive range of bespoke requirements, from initial sequence analysis and optimization to advanced purification protocols, precise bioconjugation, and sophisticated Fc Engineering for modulating effector functions. We invite you to submit your project details, and our specialists will partner with you to design and execute a production plan that perfectly aligns with your scientific goals.
Request Custom OrderTrusted By Leading Innovators









.svg)

Frequently Asked Questions
What information do I need to provide to start a project?
To initiate a project, we primarily need the amino acid sequences for the heavy and light chains (or the single chain) of your antibodies. If you have preferred codons or a specific vector you'd like to use, please provide that as well. Otherwise, our team will perform codon optimization for our high-yield expression system and clone the genes into our validated vectors to ensure optimal expression.
What is your standard expression system?
Our default platform is a highly optimized transient expression system using HEK293 cells. This system is robust and has been fine-tuned for high-yield production of a wide variety of antibody formats, offering an excellent balance of speed and productivity. For projects with specific requirements, such as particular post-translational modifications, we also offer a CHO-based expression system.
What is the typical yield I can expect from your high-throughput production service?
Yields vary depending on the antibody format, sequence complexity, and production scale. For our standard HEK293 transient expression system, we typically achieve yields of 20-100 mg/L for IgG antibodies, 10-50 mg/L for Fab fragments, and 5-30 mg/L for scFv formats. Our optimized CHO system can achieve even higher yields for certain constructs. We provide yield estimates during the quotation process and guarantee delivery of the contracted amount regardless of expression challenges.
Can I request a custom buffer or formulation?
Yes, absolutely. While our standard deliverable is formulated in PBS, we understand that downstream applications often have specific buffer requirements. We can accommodate a wide range of custom buffer requests, including different pH levels, salts, or excipients. Please specify your desired buffer composition when you request a quote so we can ensure the final product meets your exact needs.
What kind of QC data is provided?
Every antibody produced through our high-throughput service comes with a standard Quality Control (QC) package. This includes determination of the final concentration via A280 measurement and an assessment of purity and integrity by SDS-PAGE under both reducing and non-reducing conditions. For more stringent requirements, we offer advanced QC options such as SEC-HPLC for aggregation analysis, endotoxin testing for in-vivo studies, and binding assays (ELISA, SPR/BLI) to confirm activity.
How do you ensure the confidentiality and security of my sequence data?
We treat all client data with the utmost confidentiality and have robust systems in place to ensure its security. All projects are covered by a standard Confidentiality Agreement (CDA). Our data management systems are secure and access is restricted to authorized personnel only. Your sequences and project details will never be shared with any third party without your explicit consent.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.