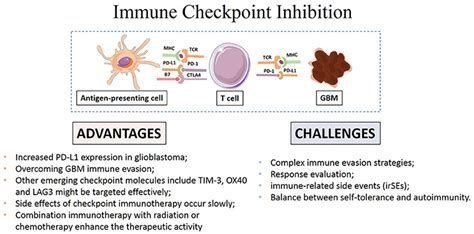
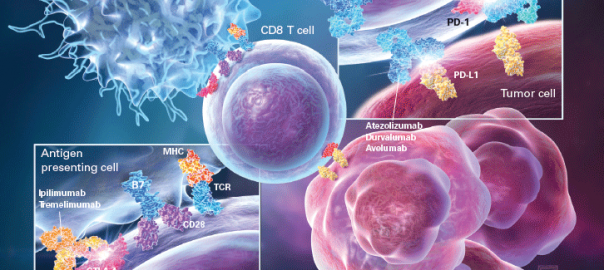
It is well known that the immune system can recognize and remove cancerous cells under normal conditions, but tumor cells will inhibit the immune system in the tumor development process, through differentRead More…
Genetech’s New Drug Rituxan Approved for the Treatment of Serious Immune Diseases as a New Breakthrough during 60 Years
A few days ago, Genentech, a Roche Group member, announced that the US FDA approved Rituxan® (rituximab) for the treatment of moderate to severe pemphigus vulgaris (PV). It is worth mentioning thatRead More…
Scientists Found Key Factors Determining Anticancer Efficacy of Immune Checkpoint Therapy
Intratumoral stimulating dendritic cells (SDCs) play a key role in stimulating cytotoxic T lymphocytes and promoting immune responses against cancer. The study of mechanisms regulating the enrichment of SDCs in tumor microenvironmentRead More…
Antibody Combo Expands Response to Checkpoint Inhibitor in Mice
Checkpoint inhibitor drugs, those that pull the brakes off the immune system, can cause dramatic improvements in cancer patients, but the benefits are far from universal. In the case of atezolizumab (Tecentriq),Read More…
Breakthrough Enables Screening Millions of Human Antibodies for New Drug Discovery
“A new article outlines a pioneering method of screening a person’s diverse set of antibodies for rapid therapeutic discovery. Antibody proteins are an important part of the human immune system that specificallyRead More…
Development History of Keytruda (Pembrolizumab)
Keytruda (pembrolizumab) is a drug developed by Merck (or MSD outside of the U.S. and Canada). It has been approved by the U.S. Food and Drug Administration (FDA) and the European Commission for the treatment of aRead More…
European Commission Approves XGEVA® (denosumab) for the Prevention of Skeletal-Related Events in Patients with Multiple Myeloma
At the beginning of this month, European Commission (EC) has approved an expanded indication for XGEVA® (denosumab) for the prevention of skeletal-related events (SRE) in adults with advanced malignancies involving bone, basedRead More…
MGH team engineers anti-inflammatory antibodies that may treat autoimmune disease
“A team of investigators has found a way to engineer antibodies within an organism, converting autoantibodies that attack ‘self’ tissues into anti-inflammatory antibodies in animal models of two autoimmune diseases.” A teamRead More…
A new weapon against bone metastasis? Princeton lab develops antibody to fight cancer
“During the past decade preclinical studies have defined many of the mechanisms used by tumours to hijack the skeleton and promote bone metastasis. This has led to the development and widespread clinicalRead More…
FDA approves emicizumab-kxwh for prevention and reduction of bleeding in patients with hemophilia A with factor VIII inhibitors
“Emicizumab (trade name Hemlibra, emicizumab–kxwh, ACE910, RO5534262) is a bispecific IgG4 mAb targeting Factors IXa and X. On November 16, 2017, the drug (emicizumab–kxwh) was approved by Food and Drug Administration (FDA) approved to preventRead More…