Intratumoral stimulating dendritic cells (SDCs) play a key role in stimulating cytotoxic T lymphocytes and promoting immune responses against cancer. The study of mechanisms regulating the enrichment of SDCs in tumor microenvironment will provide new therapeutic strategies.
To this end, researchers from the University of California, San Francisco and other institutions studied the enrichment of SDCs in human melanoma and its influencing factors, and found that the expression of Fms-related tyrosine kinase 3 ligand (FLT3LG) genes is relevant to SDC enrichment in human melanomas. The research results were recently published in Nature Medicine entitled “A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments”.
FLT3LG is mainly secreted by lymphocytes in mouse and human tumors, especially natural killer (NK) cells. NK cells can form stable conjugates with SDCs in mouse tumor microenvironment. By using genetic methods or drugs to clear NK cells in mice, the researchers found that NK cells play a key role in the enrichment of SDCs in tumors by producing FLT3LG.
The researchers also found that although anti- programmed cell death protein 1 (PD-1) immune checkpoint therapy mainly targets T cells, NK cells are usually associated with the content of protective SDCs in human tumors, the patient’s response to PD-1 therapy, and the extension of overall patient survival.
Overall, the study revealed that innately immunized SDCs, the NK cell axis, can be used as predictors of tumor immunotherapy to predict T cell targeting, and these innate immune cells are necessary to enhance T cell response to tumors. These reaction pathways are potential targets for novel immunotherapy.
About immune checkpoint therapy
Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. Inhibitory checkpoint molecules have been increasingly considered as new targets for cancer immunotherapy due to their potential for use in multiple types of cancers. Drugs or drug candidates that inhibit/block the inhibitory checkpoint molecules are sometimes known as checkpoint inhibitors; this idea is often referred to as immune checkpoint blockade, or simply checkpoint blockade.
Checkpoint inhibitor drugs have seen growth in pharmaceutical research in cancer by companies including Bristol-Myers Squibb, Merck, Roche, and AstraZeneca. Currently approved checkpoint inhibitors are ipilimumab (trade name Yervoy) that blocks the T-lymphocyte-associated antigen 4 (CTLA-4), nivolumab (trade name Opdivo) and pembrolizumab (trade name Keytruda) that block PD-1, durvalumab (trade name Imfinzi), and atezolizumab (trade name Tecentriq) that block the programmed death-ligand 1 (PD-L1).
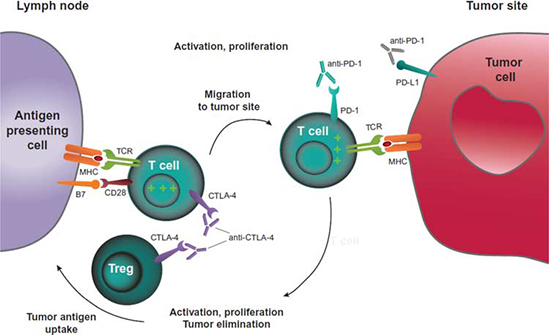
CTLA-4 and PD-1 pathway blockade. CTLA-4 blockade allows for activation and proliferation of more T-cell clones, and reduces Treg-mediated immunosuppression. PD-1 pathway blockade restores the activity of antitumor T cells that have become quiescent. A dual pathway blockade could have a synergistic effect, resulting in a larger and longer lasting antitumor immune response. MHC, major histocompatibility complex; TCR, T-cell receptor; Treg, regulatory T cell.
Reference
1. Kevin C. Barry et al, A natural killer-dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments, Nature Medicine (2018). doi: 10.1038/s41591-018-0085-8.
2. Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. American Journal of Clinical Oncology. 2016;39(1):98-106. doi:10.1097/COC.0000000000000239.