Many cancer cells never leave their original tumor tissue. Some cancer cells have evolved the ability to migrate to other tissues, but they will become dormant once they are unable to form new tumors. And the most lethal cancer cells are those that not only migrate but also thrive and multiply in distant tissues. These metastatic cancer cells are responsible for the majority of cancer-related deaths. Therefore, it is essential for researchers to understand the mechanisms behind the metastatic spread of these cells and the formation of new tumors, which may help to develop novel therapies to prevent or reverse the onset of these deadly diseases.
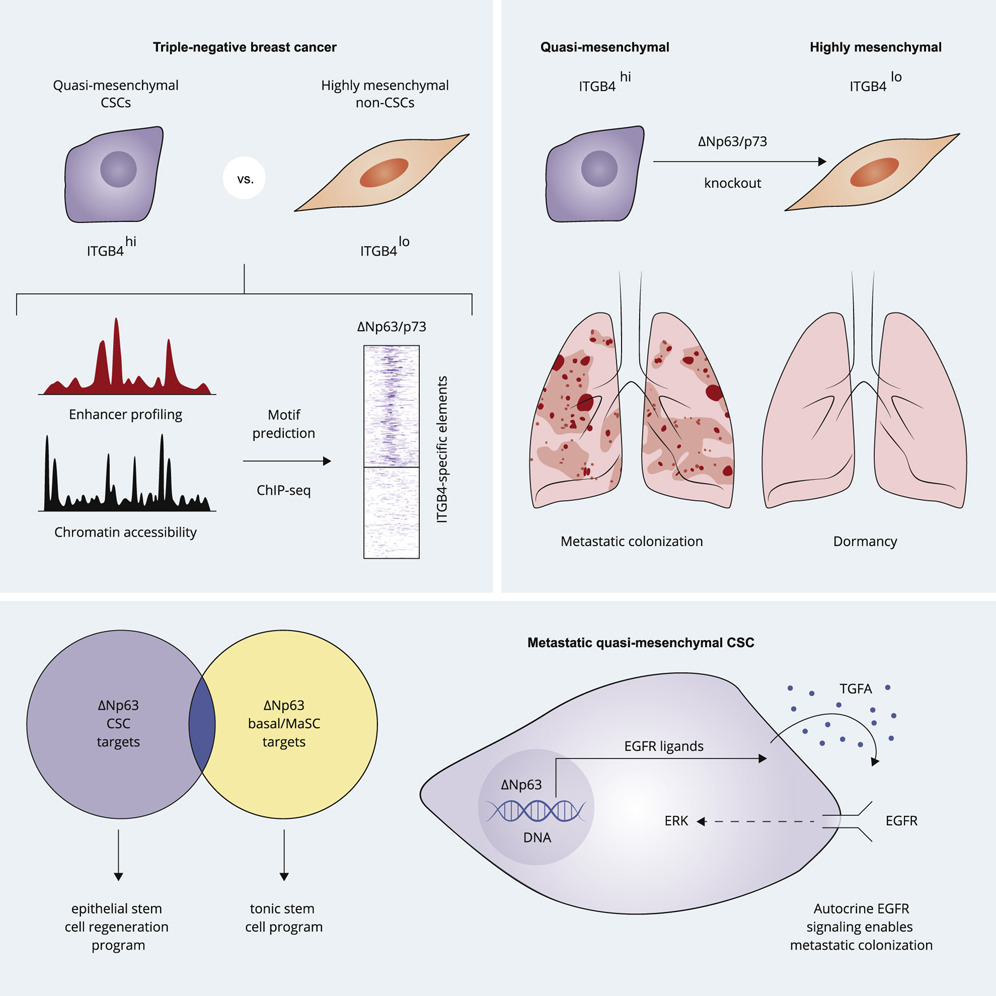
Previously, researchers have found that cancer cells are most capable of forming metastatic tumors when they are in a quasi-mesenchymal (qM) state. A study entitled “ΔNp63/p73 driving metastatic colonization by controlling a regenerative epithelial stem cell program in quasi-mesenchymal cancer stem cells” was recently published in the international journal Developmental Cell. In the study, scientists from the Whitehead Institute for Biomedical Research and other institutions identified two gene regulators that may be important for maintaining cancer cells in the qM state. The findings suggest that two molecules, ΔNp63 and p73, may drive breast cancer cells to form new tumors in mice, and the researchers have also shown how they do this.
Cells can enter the qM state by undergoing epithelial-mesenchymal transition (EMT), a developmental process that can be exploited by cancer cells, during which cells transition from an epithelial state to a more mesenchymal state, thus contributing to their becoming more mobile and invasive. Cells in the qM state can only partially transition through the EMT process, thus becoming more but not fully in the This intermediate state is ideal for metastasis, whereas cells in either the over-epithelial or over-mesenchymal states lose their ability to metastasize. As a result, Lambert et al. then wanted to learn more about how cancer stem cells capable of seeding metastases and recurrent tumors are kept in a metastasis-prone qM state. They analyzed how the regulation of gene activity occurs in these cells and identified two transcription factors (molecules that affect the activity of targeted genes) as being of great importance. One of these transcription factors is ΔNp63, which appears to most directly control the ability of cancer stem cells to maintain a qM state, while another molecule, p73, appears to have a similar function as it activates ΔNp63. When either of these transcription factors is inactivated, cancer stem cells transition to the distal end of the EMT spectrum and are unable to metastasize.
Next, the researchers analyzed which genes are regulated by ΔNp63 in cancer stem cells. They expected to find a pattern of gene regulation similar to that found in healthy breast stem cells. Instead, they discovered a specific pattern, perhaps similar to that observed in cells involved in wound healing and regeneration. Notably, ΔNp63 stimulates EGFR signaling, which can be used to promote rapid cell multiplication during the wound healing process. “Although this is not what we expected, it is of great significance because the specialized process requires more aggressive proliferation, and metastatic cancer cells need the properties of stem cells (such as the ability to self-renew and differentiate into different cell types) and the ability to multiply their numbers to grow into new tumors,” said Lambert.
The results of this study may explain why qM cells have such a unique ability to metastasize and why it is only in the qM state that the cells can strongly stimulate EGFR signaling and promote their own proliferation. It may also provide researchers with some mechanistic understanding of the qM state that drives metastatic tumor growth. The researchers expect that these insights may eventually facilitate the development of novel therapies for preventing cancer metastasis and help them further understand the role of the ΔNp63 molecule. This work also elucidates a possible link between ΔNp63 and the activation of dormant cancer cells, which can travel to new tissues but fail to proliferate upon arrival. These cells are often considered time bombs as they can awaken at any time. Ultimately, the researchers hope that further research will be conducted to uncover the mechanisms that facilitate the ability of dormant cells to grow into new tumors, as well as to help understand the molecular mechanisms by which cancer metastasis occurs.
Reference
1. Lambert, Arthur W., et al. “ΔNp63/p73 drive metastatic colonization by controlling a regenerative epithelial stem cell program in quasi-mesenchymal cancer stem cells.” Developmental Cell 57.24 (2022): 2714-2730.