Immunotherapy has revolutionized the treatment of many cancer types. However, for reasons that remain poorly understood, not all patients respond equally from these potent therapies.
In a recent study, researchers from Harvard Medical School and the Dana-Farber Cancer Institute pointed out that one powerful factor affecting the outcome of cancer immunotherapy treatments appears to be the patient’s gut flora—the trillions of microbes that live in the human gut. The findings were published online May 3, 2023, in the journal Nature titled “Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy resistance”.
The study, done in mice, demonstrates how gut microbes can enhance the body’s response to PD-1 checkpoint inhibitor therapy, a common type of immunotherapy which is currently used to treat 25 different types of cancer.
The study found that specific gut bacteria can affect the activity of two immune molecules, programmed cell death-ligand 2 (PD-L2) and repulsive guidance molecule B (RGMb), and how they interact with each other. The study also showed that blocking the activity of either molecule or the interaction between them could enhance the response to cancer immunotherapy and maximize the body’s ability to detect and destroy cancer cells.
Arlene Sharpe, co-corresponding author of the paper and chair of the Department of Immunology at the Blavatnik Institute at Harvard Medical School, said, “Our study shows that treatment with antibodies that block the interaction of PD-L2 with RGMb can unblock cancer-fighting T cells, allowing them to eradicate tumors.”
The new study also identifies the RGMb molecule as a previously unknown accomplice in the body’s ability to disrupt the discovery and destruction of tumors. RGMb, known primarily for its role in nervous system development, is also found on the surface of cancer-fighting T cells. However, until then, no one knew that it played a role in regulating T-cell responses to cancer immunotherapy.
Joon Seok Park, co-first author of the paper and a postdoctoral fellow in immunology in the Sharpe lab, said, “Our findings provide key clues to a complex puzzle, and in doing so, suggest specific ways to enhance the efficacy of cancer immunotherapy and improve patient prognosis. We propose a novel approach to overcome this disease’s resistance to current cancer immunotherapy by understanding the gut bacteria that help our immune system fight cancer.”
How cancer evades immune detection and destruction
The key to cancer’s survival and spread is its ability to evade the body’s immune defenses. Beginning in the 1990s, Sharpe and Gordon Freeman, professors of medicine at the Dana-Farber Cancer Institute, conducted some key early research efforts to shed light on how cancer does this.
Sharpe and Freeman’s work focused on two molecules that reside on the surface of immune cells—PD-L1 and PD-L2—and their research has shown that T-cell activity is controlled when PD-L1 or PD-L2 interacts with another molecule called PD-1 on the surface of T cells. Under normal circumstances, this interaction acts as a brake on T cells to ensure that they do not mistakenly attack the body’s own cells and tissues.
Sharpe, Freeman, and others found that cancer uses this very safety mechanism to evade detection and destruction by T cells. Cancer cells do this by expressing PD-L1 and PD-L2 on their surfaces, binding to PD-1, and controlling T cells. Cancer immunotherapies that block the interaction of PD-1 with PD-L1 or PD-L2 release T cells from attacking the cancer, and are thus called immune checkpoint inhibitors.
This type of immunological strategy is being employed for 25 cancer types and has revolutionized cancer care, although it has not benefited some certain portion of patients. Scientists have been attempting to understand the reasons for this since the advent of these treatments.
The interaction between the immune system and gut flora has been the focus of research by Dennis Kasper, professor of immunology at the Blavatnik Institute at Harvard Medical School, for many years. His lab has identified not only the regulatory mechanisms, but also the specific microbial molecules and microbial enzymes responsible for regulating the immune system.
The concept that gut microbes may influence cancer immunotherapy is not entirely new. Recent studies have uncovered tantalizing clues about the role of gut microbes in the outcome of immunotherapy treatments. Until then, however, a key question remains unanswered: How does this happen?
A new participant enters the field
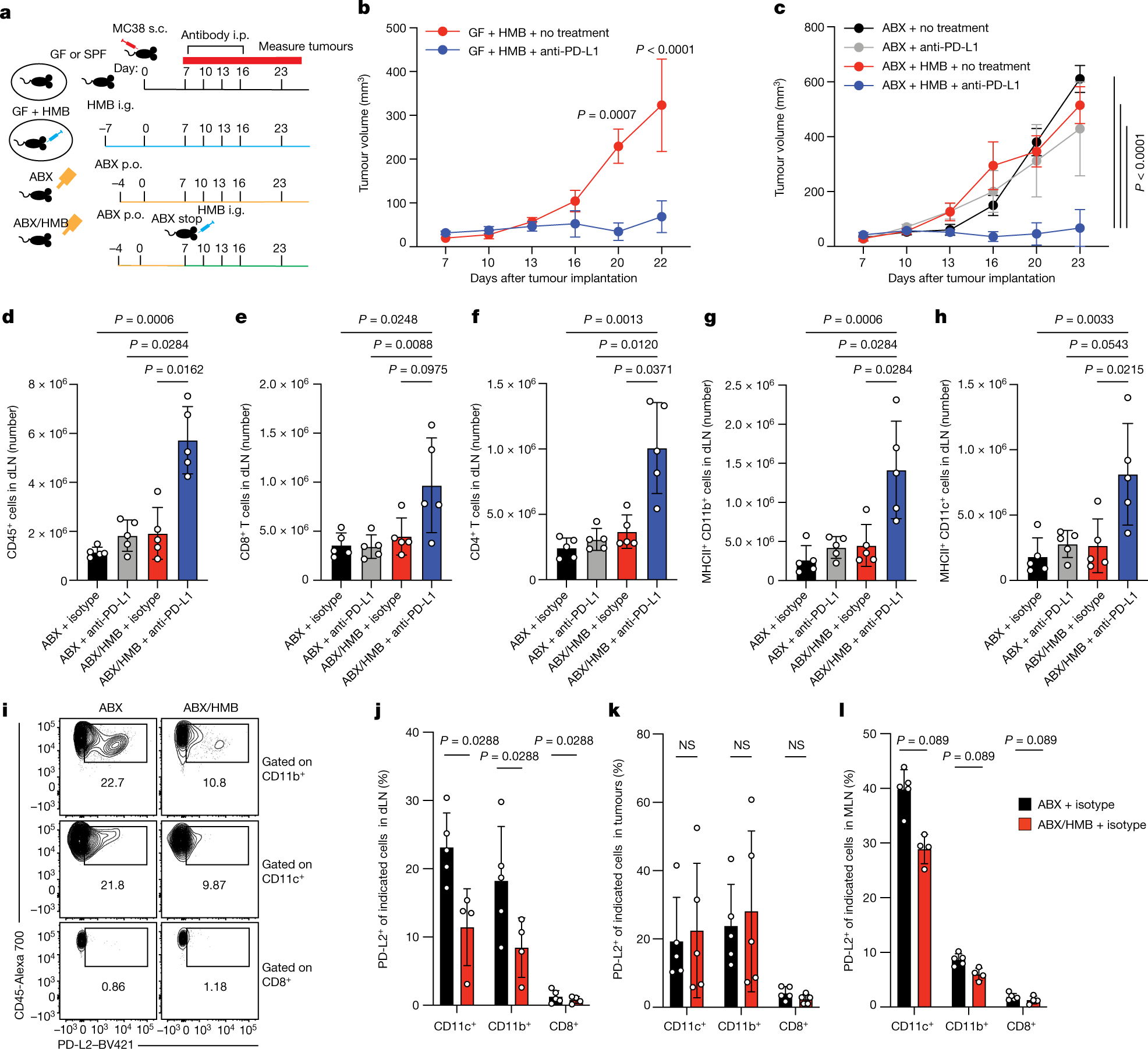
In this new study, the authors used mice whose colons had been implanted with the intestinal flora of cancer patients as study subjects. Some of these patients responded well to the immunotherapy, while others gained little benefit. The response of these mice to immunotherapy mimicked the therapeutic response of humans with gut microbes inhabiting their intestines.
These authors compared the immune systems of these two groups of mice and found significant differences in the various immune cells involved in cancer detection and destruction. The data implies that the gut flora altered the activity of these immune cells and thus the responsiveness to immunotherapy.
Mice inoculated with gut microbes from patients who responded well to cancer immunotherapy showed lower levels of PD-L2 on the surface of an immune cell class called antigen-presenting cells. These cells are critical in launching the body’s immune defenses. They accomplish this by scouring the body for pathogens or tumors and delivering these alien or aberrant proteins to T cells for elimination. In contrast, mice inoculated with gut microbes from patients who responded poorly to immunotherapy had higher levels of PD-L2.
To investigate the effect of specific gut microbes, the researchers subjected these mice to a broad-spectrum antibiotic treatment that killed gut bacteria. The antibiotic-treated mice did not respond to PD-1 molecule-blocking immunotherapy. These mice, on the other hand, had large amounts of PD-L2, another “molecular brake” that normally works through PD-1. Mice with a robust response to the same therapy exhibited lower levels of PD-L2.
Curious that PD-1 inhibitor did not work, these authors hypothesized that PD-L2 operates as a “brake” on T cells not through PD-1 alone but through another molecular accomplice. They turned their attention to RGMb, which had previously been shown by the Freeman lab to control immunological tolerance in the lung alongside PD-L2.
When these authors treated mice that did not respond to anti-PD-1 therapy alone with RGMb antibody, these mice experienced an increase in cancer-fighting T cells and rapid overall recovery.
“The interplay between gut flora and immune cells in the anti-cancer response is becoming clearer,” Freeman said, “and with the identification of RGMb as a molecular accomplice to PD-L2, we have an alternative target for cancer immunotherapy.”
Further analysis revealed that the interaction between RGMb and PD-L2 depends on the composition of gut microbes. They found that certain gut microbes can influence the levels of both molecules.
Cancer-bearing mice inoculated with certain gut microbes had six-fold lower levels of RGMb on the surface of their T cells and responded to anti-PD-L1 or anti-PD-1 immunotherapy than mice with no microbes in their gut. In contrast, mice with eliminated gut microbiota did not respond to these treatments and had higher levels of RGMb on the surface of their T cells, particularly those that infiltrated into the tumor.
Similarly, mice inoculated with gut flora from patients who did not respond well to treatment had higher levels of RGMb in their intestines, implying that patients who do not respond well to cancer immunotherapy have higher levels of RGMb on the surface of their T cells, which in turn interferes with the anti-tumor response of immune cells in vivo.
Inactivating PD-L2 or RGMb is sufficient to maintain the anti-tumor activity of T cells and ensure a robust response to PD-L1 and PD-1 therapies. Notably, blocking PD-L2 activity resulted in a strong antitumor response in mice receiving dendritic cell therapy. This observation suggests that modulating PD-L2 activity may hold promise for improving responses to immunotherapy for varieties of cancers.
Gut microbes regulate the body’s immune responses
These authors found that by altering the composition of the gut flora in different groups of mice, one gut microbe, C. cateniformis, suppressed levels of PD-L2 and made immunotherapy more effective in cancer-stricken mice.
Given that the human gut contains thousands of species of bacteria, these investigators speculated that this microbe is not the only one capable of influencing antitumor immunity.
This finding implies that specific microbial molecules can be harnessed in the form of small molecule drugs that enhance the immune system’s ability to control cancer. Such treatments may complement or replace standard antibody-based cancer immunotherapies.
Sharpe noted that the small molecule approach has the advantage of being less expensive to develop and store, as well as easier to get into the body. Small molecule drugs are typically administered orally, whereas cancer immunotherapy is administered as intravenous antibodies.
While their study exposes a critical piece of the jigsaw, the authors caution that it could be just one of multiple ways in which the immune system and gut flora interact in cancer.
“This may be just the beginning of the story,” according to Francesca Gazzaniga, co-first author of the paper and a former postdoctoral fellow in Kasper’s lab. “The complexity of cancer, the immune system, and the gut microbiome are each astounding, but when they combine, the interactions become exponentially more complex.”
“In general, the gut microbiome may influence cancer immunity in many other ways, particularly cancer immunotherapy.” Kasper added. “We have identified a completely new way to study how the gut flora affects not only the efficacy of cancer therapy, but also how it affects cancer immunity.”
Reference
1. Park, Joon Seok, et al. “Targeting PD-L2–RGMb overcomes microbiome-related immunotherapy resistance.” Nature (2023): 1-9.