Chemokines and their receptors have been shown to play crucial roles in regulating tumor growth, progression, metastasis, and response to immunotherapy. Initially known for their role as chemoattractants in guiding leukocyte migration, emerging evidence suggests that cheymokines can regulate various functions in a broader range of cell types, including cancer cells.
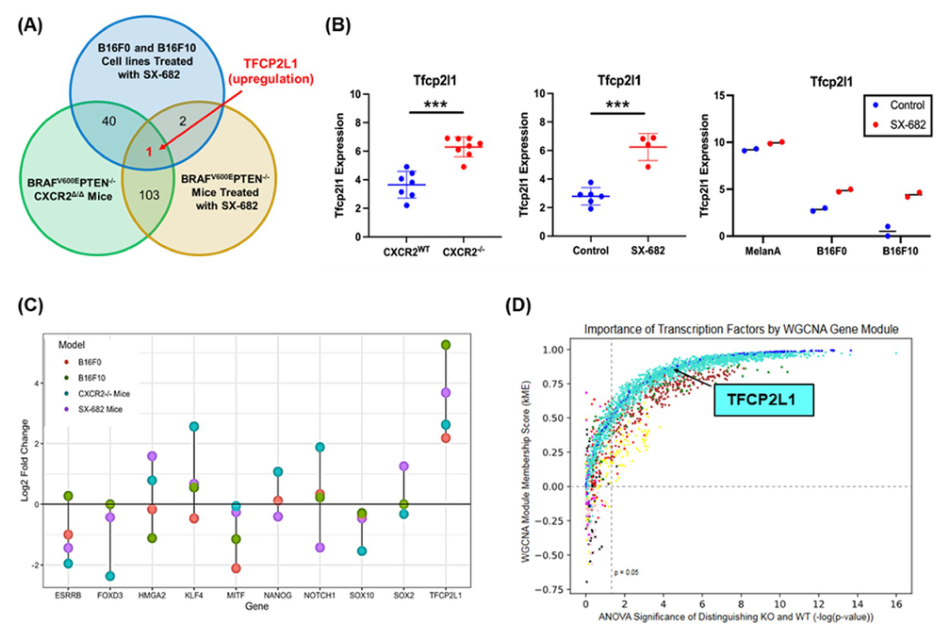
In a recent study titled “CXCR2 expression during melanoma tumorigenesis controls transcriptional programs that facilitate tumor growth” published in Molecular Cancer, researchers from the U.S. Department of Veterans Affairs at TVHS have provided novel mechanistic insights into how the loss of CXCR2 expression/activity in melanoma tumor precursor cells leads to reduced tumor burden and the creation of an anti-tumor immune microenvironment. This mechanism involves increased expression of the tumor-suppressive transcription factor Tfcp2l1, along with changes in gene expression related to growth regulation, tumor suppression, stemness, differentiation, and immune modulation. These changes in gene expression are consistent with reduced activation of key growth regulatory pathways, including AKT and mTOR.
Although the critical role of the CXCR2 chemokine receptor in tumor growth and treatment response is known, a direct link between CXCR2 expression in tumor precursor cells during tumor initiation has not yet been established.
To investigate the role of CXCR2 in melanoma tumorigenesis, the researchers established tamoxifen-induced tyrosinase promoter-driven BRAFV600E/PTEN-/-/CXCR2-/- and NRASQ61R/INK4a-/-/CXCR2-/- melanoma mouse models. Additionally, they studied the effects of the CXCR1/CXCR2 antagonist SX682 in BRAFV600E/Pten-/- and NRASQ61R/INK4a-/- mice and melanoma cell lines.
The researchers used RNAseq, mMCP counter, ChIPseq, qRT-PCR, flow cytometry, and reverse-phase protein array (RPPA) to explore the potential mechanisms by which CXCR2 influences melanoma tumorigenesis in these mouse models.
During melanoma initiation, genetic deletion of CXCR2 or pharmacological inhibition of CXCR1/CXCR2 resulted in critical changes in gene expression, leading to reduced tumor incidence/growth and enhanced anti-tumor immunity. Interestingly, upon CXCR2 ablation, the key tumor-suppressive transcription factor Tfcp2l1 was the only significantly induced gene with a log2 fold change greater than 2 across these three distinct melanoma models.
These findings support the combination of CXCR1/CXCR2 antagonists with immunotherapy in melanoma patients. Consistent with this concept, it has been demonstrated that the antagonism of CXCR2 upregulates PD-L1 expression and enhances melanoma cell response to PD-1 blockade.
Moreover, a clinical trial (NCT03161431) of CXCR1/CXCR2 antagonists combining with anti-PD-1 therapy is currently underway for the treatment of melanoma, which will be crucial to identify patient subgroups most likely to respond to this combination therapy and devise optimal response strategies.
Reference
1. Yang, Jinming, et al. “CXCR2 expression during melanoma tumorigenesis controls transcriptional programs that facilitate tumor growth.” Molecular Cancer 22.1 (2023): 92.