Anti-Human CTLA4 Recombinant Antibody (TAB-067)
CAT#: TAB-067
Recombinant monoclonal antibody to CTLA4. It is a drug used for the treatment of melanoma, a type of skin cancer. It is a U.S. Food and Drug Administration (FDA) approved human monoclonal antibody, and works by activating the immune system by targeting CTLA-4.
Cytotoxic T lymphocytes (CTLs) can recognize and destroy cancer cells. However, there is also an inhibitory mechanism that interrupts this destruction. It turns off this inhibitory mechanism and allows CTLs to continue to destroy cancer cells.



Specifications
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Human
- Derivation
- Human
- Type
- IgG1 - kappa
- Specificity
- Tested positive against native human antigen.
- Species Reactivity
- Human
- Applications
- WB, FuncS, IF, Neut, ELISA, FC, IP, IHC
- MW
- 148,634.914 g/mol
- Related Disease
- Melanoma, metastatic
Product Property
- Purity
- >97%, by SDS-PAGE under reducing conditions and visualized by silver stain.
- Storage
- 4°C. For long term storage, aliquot and store at -20°C. Repeated thawing and freezing must be avoided.
Applications
- Application Notes
- The CTLA4 antibody has been reported in applications of WB, FuncS, IF, Neut, ELISA, FC, IP, IHC.
Target
- Alternative Names
- Cytotoxic T-Lymphocyte Associated Protein 4; Insulin-Dependent Diabetes Mellitus 12; Celiac Disease 3; CTLA-4; CD152; Ligand And Transmembrane Spliced Cytotoxic T Lymphocyte Associated Antigen 4; Cytotoxic T Lymphocyte Associated Antigen 4 Short Spliced Form; Cytotoxic T-Lymphocyte-Associated Serine Esterase-4; Cytotoxic T-Lymphocyte-Associated Antigen 4; Cytotoxic T-Lymphocyte Protein 4
- Gene ID
- 1493
- UniProt ID
- P16410
Customer Review
There are currently no Customer reviews or questions for TAB-067. Click the button above to contact us or submit your feedback about this product.
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Related Diseases
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
Protocol & Troubleshooting
We have outlined the assay protocols, covering reagents, solutions, procedures, and troubleshooting tips for common issues in order to better assist clients in conducting experiments with our products. View the full list of Protocol & Troubleshooting.
See other products for "CTLA4"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| DrMAB-986 | Recombinant Mouse Anti-Human CTLA4 Antibody F(ab')2 Fragment, clone ANC152.2/8H5 | FC, EIA, FX, Bios | F(ab')2 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| DrMAB-987 | Mouse Anti-CTLA4 Recombinant Antibody (clone ANC152.2/8H5); Fab Fragment | FC, ELISA | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-206 | Anti-Human CTLA4 Recombinant Antibody (Ticilimumab (Tremelimumab)) | WB, IF, IP, Neut, FuncS, ELISA, FC | IgG2 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-G015E | Anti-CTLA4 immunotoxin (scFv)-PE | Cytotoxicity assay, Function study |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-G015S | Anti-CTLA4 immunotoxin (scFv)-Sap | Cytotoxicity assay, Function study |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-G015M | Anti-CTLA4 immunotoxin (scFv)-Mel | Cytotoxicity assay, Function study |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-195CL | Mouse Anti-CTLA4 Recombinant Antibody (TAB-195CL) | ELISA | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-083MZ | Anti-Human CTLA4 Recombinant Antibody (TGN2122.H) | ELISA, FC | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-084MZ | Anti-Human CTLA4 Recombinant Antibody (TGN2422.H) | ELISA, FC | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-085MZ | Anti-Human CTLA4 Recombinant Antibody (TGN2122.C) | ELISA, FC | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-086MZ | Anti-Human CTLA4 Recombinant Antibody (TGN2422.C) | ELISA, FC | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-087MZ | Mouse Anti-CTLA4 Recombinant Antibody (TAB-087MZ) | ELISA | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-088MZ | Human Anti-CTLA4 Recombinant Antibody (TAB-088MZ) | ELISA | Human IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-089MZ | Mouse Anti-CTLA4 Recombinant Antibody (TAB-089MZ) | ELISA | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-090MZ | Mouse Anti-CTLA4 Recombinant Antibody (TAB-090MZ) | ELISA | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-091MZ | Human Anti-CTLA4 Recombinant Antibody (TAB-091MZ) | ELISA | Human IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-092MZ | Human Anti-CTLA4 Recombinant Antibody (TAB-092MZ) | ELISA | Human IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-093MZ | Anti-Human CTLA4 Recombinant Antibody (Mu-26B) | ELISA |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-085MZ-S(P) | Anti-Human CTLA4 Recombinant Antibody scFv Fragment (TGN2122.C) | ELISA, FC | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-086MZ-S(P) | Anti-Human CTLA4 Recombinant Antibody scFv Fragment (TGN2422.C) | ELISA, FC | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-087MZ-S(P) | Mouse Anti-CTLA4 Recombinant Antibody; scFv Fragment (TAB-087MZ-S(P)) | ELISA | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-085MZ-F(E) | Anti-Human CTLA4 Recombinant Antibody Fab Fragment (TGN2122.C) | ELISA, FC | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1734MZ | Recombinant Mouse Anti-Human CTLA4 Antibody (clone COJ4) | FC, ICC, IHC-Fr | Mouse antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-567CQ | Mouse Anti-CTLA4 Recombinant Antibody (NEUT-567CQ) | FC, FuncS, Neut, IP, WB | Mouse IgG2a, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-568CQ | Mouse Anti-CTLA4 Recombinant Antibody (clone L3D10) | FC, Block | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-569CQ | Mouse Anti-CTLA4 Recombinant Antibody (clone AS32) | WB, Neut | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-570CQ | Human Anti-CTLA4 Recombinant Antibody (clone YC030) | Neut | Human IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-571CQ | Mouse Anti-CTLA4 Recombinant Antibody (clone BN13) | Block, FC | Mouse IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-572CQ | Hamster Anti-Ctla4 Recombinant Antibody (clone UC10-4B9) | FC, Block, ELISA, IP | Hamster IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-573CQ | Rat Anti-Ctla4 Recombinant Antibody (clone 63828) | WB, FC, Neut | Rat IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-577CQ | Hamster Anti-Ctla4 Recombinant Antibody (clone MAB0835) | Block, FC | Hamster IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-578CQ | Hamster Anti-Ctla4 Recombinant Antibody (NEUT-578CQ) | Block | Hamster IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-579CQ | Hamster Anti-Ctla4 Recombinant Antibody (clone 9H10) | FC, Block | Hamster IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-0855 | Hi-Affi™ Rabbit Anti-CTLA4 Recombinant Antibody (clone DS855AB) | WB | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0051-YC | Human Anti-CTLA4 Recombinant Antibody (clone 10D1) | ELISA, Cyt, Block | Human IgG, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0051-YC-S(P) | Human Anti-CTLA4 Recombinant Antibody (clone 10D1); scFv Fragment | ELISA, Cyt, Block | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0051-YC-F(E) | Human Anti-CTLA4 Recombinant Antibody (clone 10D1); Fab Fragment | ELISA, Cyt, Block | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-006LC | Hamster Anti-Ctla4 Recombinant Antibody (clone YC082) | ELISA, FC | Hamster IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-061LC | Human Anti-CTLA4 Recombinant Antibody (MOB-061LC) | ELISA, WB, FuncS | Human IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0157-FY | Human Anti-CTLA4 Recombinant Antibody (HPAB-0157-FY) | Activ | Humanized IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0157-FY-S(P) | Human Anti-CTLA4 Recombinant Antibody; scFv Fragment (HPAB-0157-FY-S(P)) | Activ | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0157-FY-F(E) | Human Anti-CTLA4 Recombinant Antibody; Fab Fragment (HPAB-0157-FY-F(E)) | Activ | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-N0343-YC-S(P) | Human Anti-CTLA4 Recombinant Antibody; scFv Fragment (HPAB-N0343-YC-S(P)) | FC, ELISA | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-N0343-YC-F(E) | Human Anti-CTLA4 Recombinant Antibody; Fab Fragment (HPAB-N0343-YC-F(E)) | FC, ELISA | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0749LY-S(P) | Human Anti-CTLA4 Recombinant Antibody; scFv Fragment (HPAB-0749LY-S(P)) | FuncS, ELISA | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0750LY-S(P) | Human Anti-CTLA4 Recombinant Antibody; scFv Fragment (HPAB-0750LY-S(P)) | FuncS, ELISA | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-067 | Afuco™ Anti-CTLA4 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-067) | FuncS, IF, Neut, ELISA, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-206 | Afuco™ Anti-CTLA4 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-206) | IF, IP, Neut, FuncS, ELISA | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP860-YC | Camelid Anti-CTLA4 Recombinant Antibody; VHH Fragment (HPAB-AP860-YC) | ELISA, Block | Camel VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP861-YC | Camelid Anti-CTLA4 Recombinant Antibody; VHH Fragment (HPAB-AP861-YC) | ELISA, Block | Camel VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP862-YC | Camelid Anti-CTLA4 Recombinant Antibody; VHH Fragment (HPAB-AP862-YC) | ELISA, Block | Camel VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP863-YC | Camelid Anti-CTLA4 Recombinant Antibody; VHH Fragment (HPAB-AP863-YC) | ELISA, Block | Camel VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP864-YC | Camelid Anti-CTLA4 Recombinant Antibody; VHH Fragment (HPAB-AP864-YC) | ELISA, Block | Camel VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0724-XY22 | Mouse Anti-CTLA4 Agonistic Antibody (VS-0724-XY22) | WB, Agonistic assays | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0424-XY73 | AbPlus™ Anti-CTLA4 Magnetic Beads (14D3) | IP, Protein Purification |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY92 | CytoStream™ Mouse Anti-CTLA4 Recombinant Antibody (clone BNI3) | FC | Mouse IgG2a, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY93 | CytoStream™ Armenian Hamster Anti-CTLA4 Recombinant Antibody (clone UC10-4F10-11) | FC, IP | Armenian Hamster IgG1, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0325-FY63 | Human Anti-CTLA4 (clone JMW-3B3) scFv-Fc Chimera | ELISA | Human IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0425-YC558 | Recombinant Anti-CTLA4 Vesicular Antibody, EV Displayed (VS-0425-YC558) | ELISA, FC, SPR, Neut, Cell-uptake |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY1763 | Anti-CTLA4 Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY1764 | Anti-Mouse CTLA4 Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC87 | SmartAb™ Recombinant Anti-CTLA4 pH-dependent Antibody (VS-0825-YC87) | WB, IF, Neut, ELISA, FC, IP | Human IgG1 kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1025-YC10 | Anti-CTLA4 Antibody Prodrug, Protease Activated (Ticilimumab) | ISZ, Cyt, FuncS |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1025-YC196 | Anti-CTLA4 Antibody Prodrug, Protease Activated (10D1) | ISZ, Cyt, FuncS |
Popular Products

Application: WB, ELISA, IP, FC, FuncS, Neut, IF

Application: FC, IP, ELISA, Neut, FuncS, IF, IHC

Application: WB, ELISA, FC, IP, FuncS, IF, Neut

Application: IF, IP, Neut, FuncS, ELISA, FC, WB

Application: WB, ELISA, FC, IP, FuncS, IF, Neut

Application: ELISA, FC, IP, FuncS, IF, Neut, ICC

Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: FC, IP, ELISA, Neut, FuncS, IF, IHC

Application: Neut, ELISA, FuncS

Application: ELISA, IP, WB, IHC, IF, FuncS

Application: FuncS, Inhib, IP, ELISA

Application: ELISA, SPR, Inhib, FuncS
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.






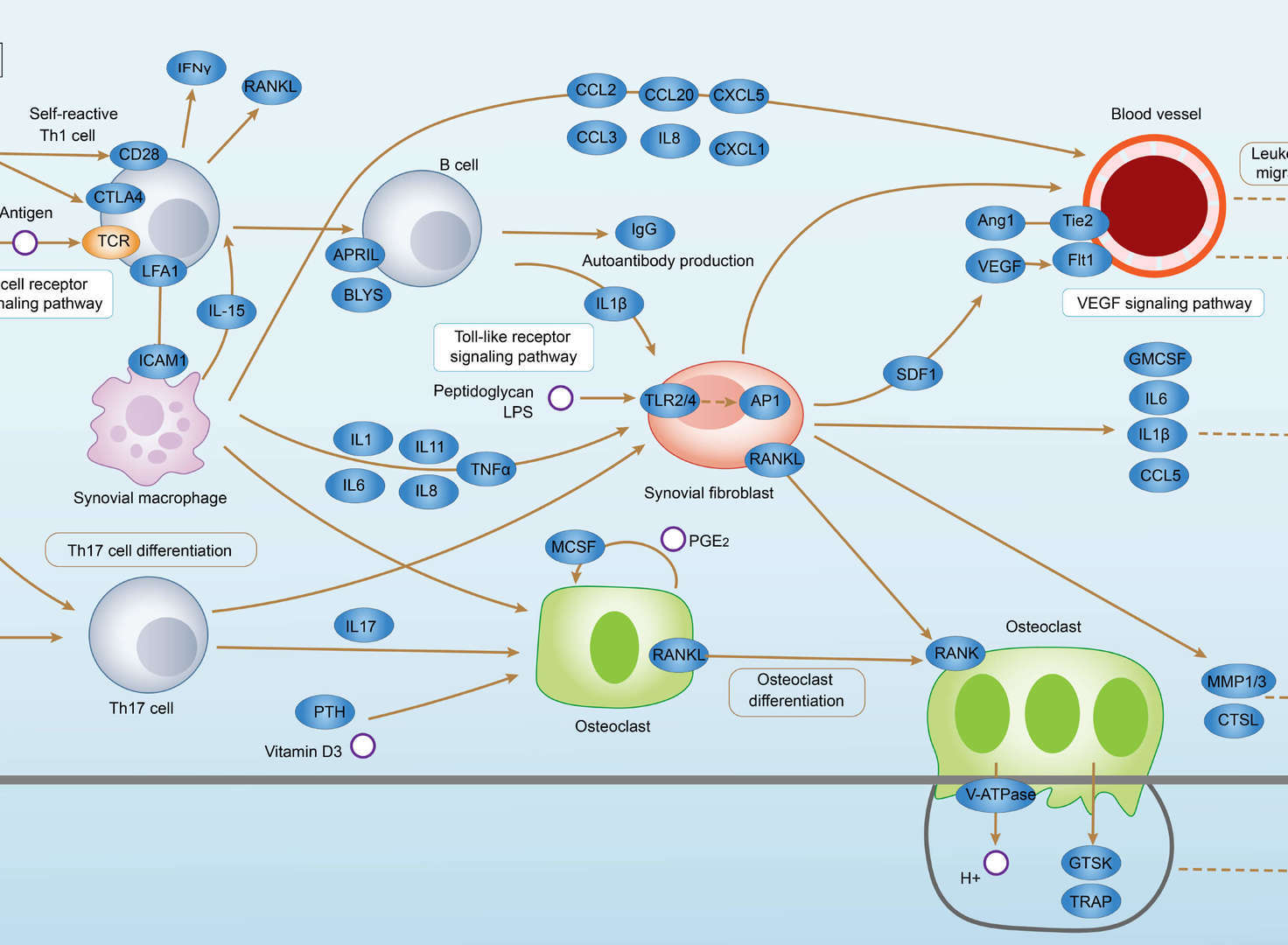 Rheumatoid Arthritis
Rheumatoid Arthritis










