

Fatty Acid Synthesis
Fatty acid synthesis is an essential biochemical process through which cells convert acetyl-CoA, derived from carbohydrates, proteins, and lipids, into fatty acids, fundamental components of lipid molecules that are crucial for various cellular functions. This process primarily occurs in the cytoplasm and is catalyzed by a series of enzymes, with acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) playing key roles. ACC first converts acetyl-CoA into malonyl-CoA, which then serves as the substrate for FAS, a multi-enzyme complex that elongates the carbon chain step-by-step until the fatty acid reaches its final length. The regulation of fatty acid synthesis involves a sophisticated network of hormonal and nutritional signals that ensure its activity corresponds with the energy needs and supply of the cell. Insulin, for instance, promotes fatty acid synthesis, signaling a state of energy abundance, whereas glucagon inhibits this process, indicating energy scarcity. Moreover, the synthesis pathway is tightly linked to the cellular energy status, monitored by AMP-activated protein kinase (AMPK), which downregulates fatty acid synthesis under energy-deficient conditions by inhibiting ACC. Fatty acids produced through this pathway are pivotal for numerous biological functions—they serve as key components of cell membranes, provide energy storage in the form of triglycerides, act as signaling molecules, and are precursors for other biologically important lipids. Disruptions in fatty acid synthesis can lead to severe metabolic disorders, including type 2 diabetes, obesity, and fatty liver disease..
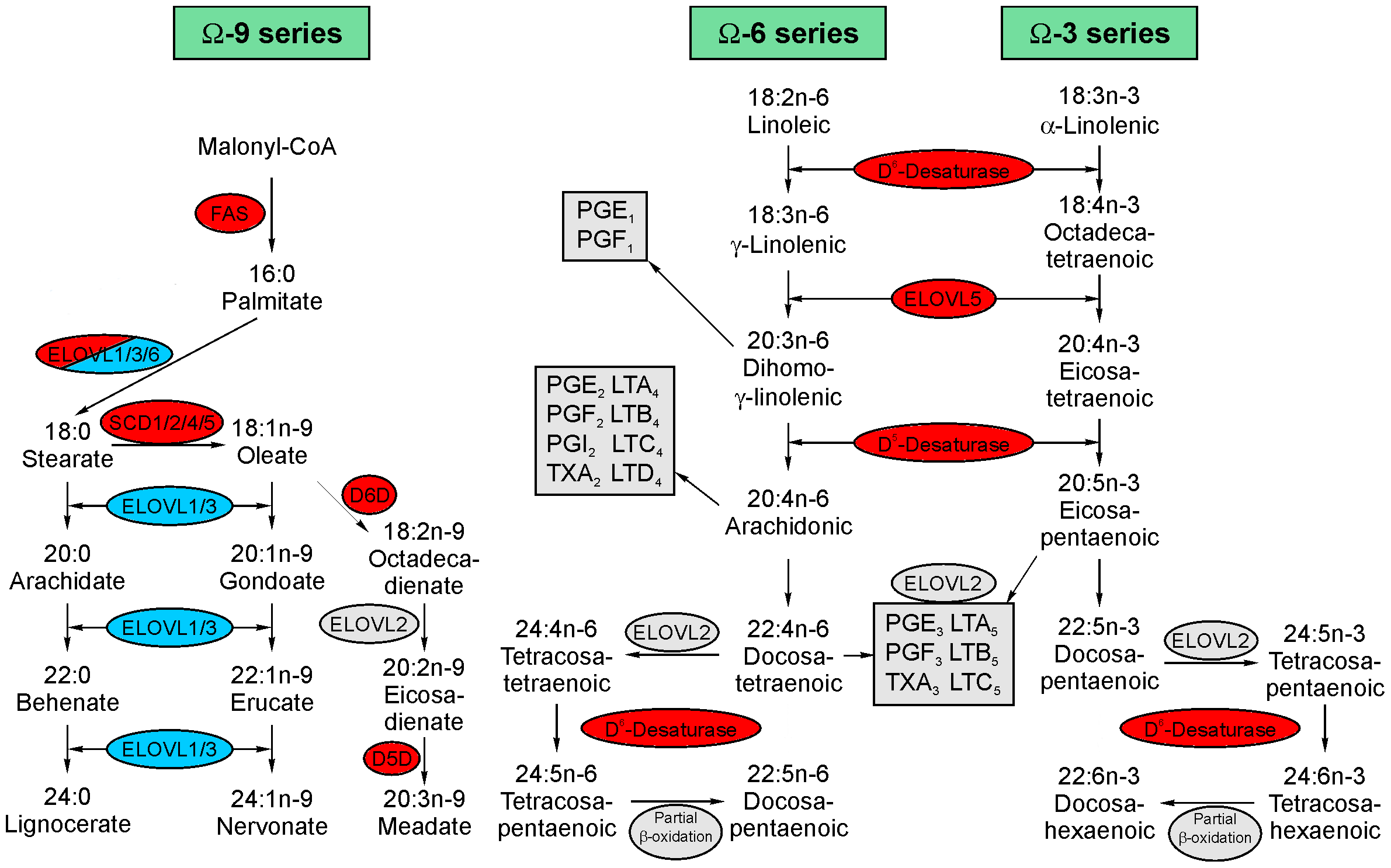 Figure 1 Schematic representation of fatty acid synthesis pathways. (Wallner, 2014)
Figure 1 Schematic representation of fatty acid synthesis pathways. (Wallner, 2014)
Representative Targets in Fatty Acid Synthesis
FASN
FASN, or fatty acid synthase, is a multifunctional enzyme complex critical in the biosynthesis of long-chain fatty acids. This enzyme catalyzes the synthesis of palmitate (C16:0) from acetyl-CoA and malonyl-CoA in the presence of NADPH, essentially constructing fatty acids through a series of condensation, reduction, and dehydration reactions. FASN is highly active in liver and adipose tissues where it plays a significant role in lipid metabolism, converting excess carbohydrates into fatty acids for storage as triglycerides. In addition to its metabolic functions, FASN's role extends into various other cellular processes, including cell signaling, proliferation, and survival. The enzyme's activity is crucial for the generation of lipid-based signaling molecules and for the modification of proteins through lipidation, which influences membrane composition and protein function. Due to these essential roles, FASN is tightly regulated by nutritional and hormonal signals, reflecting the body's metabolic state. Importantly, aberrant expression and activity of FASN are observed in numerous diseases, notably in cancer. Many tumors exhibit overexpression of FASN, which supports the high demands for membrane synthesis in rapidly proliferating cancer cells and contributes to enhanced growth and survival. FASN has therefore been identified as a potential oncogenic driver and a target for anticancer therapy. Inhibitors of FASN are currently being explored as therapeutic agents, aiming to exploit the dependency of certain cancers on de novo fatty acid synthesis for their progression and survival.



ACLY
ACLY, or ATP Citrate Lyase, is a crucial enzyme that links carbohydrate metabolism to fat synthesis. This enzyme catalyzes the conversion of citrate and coenzyme A (CoA) into acetyl-CoA and oxaloacetate, utilizing ATP in the process. Acetyl-CoA is a vital metabolic intermediate that not only serves as a building block for fatty acid synthesis but is also important in the biosynthesis of cholesterol and other acetyl-containing compounds. ACLY is predominantly active in the liver, adipose tissue, and other tissues involved in lipid biosynthesis. The enzyme plays a pivotal role in metabolic pathways by providing acetyl-CoA for lipogenesis during periods when carbohydrates are abundant, essentially converting excess glucose into stored fats. The activity of ACLY is thus tightly regulated by nutritional status, hormonal signals, and cellular energy levels. Phosphorylation and allosteric regulation are key mechanisms controlling its activity, with insulin promoting and AMP-activated protein kinase (AMPK) inhibiting its function. In addition to its metabolic roles, ACLY is increasingly recognized for its contribution to pathological states, particularly in metabolic diseases like obesity and diabetes, and in cancer. In cancer cells, ACLY facilitates the production of acetyl-CoA required for rapid cell growth and proliferation, supporting the synthesis of membrane lipids and other macromolecules. Elevated levels of ACLY are often found in various cancers and are associated with poor prognosis.


CHKB
CHKB, or Choline Kinase Beta, is an enzyme that plays a crucial role in the biosynthesis of phosphatidylcholine (PC) via the Kennedy pathway, one of the major pathways for the synthesis of phospholipids in cells. Phosphatidylcholine is a fundamental component of cell membranes and is essential for maintaining the structural and functional integrity of cells. CHKB catalyzes the phosphorylation of choline to form phosphocholine, which is a key intermediate step in the production of phosphatidylcholine. CHKB is particularly significant in muscle and neuronal tissues, where the demand for membrane synthesis and repair is high. The enzyme's activity contributes to various cellular processes, including cell signaling, lipid metabolism, and membrane dynamics. Mutations or dysregulation in CHKB have been associated with various human diseases, notably muscular dystrophies and neurodegenerative disorders. For instance, mutations in the CHKB gene have been linked to congenital muscular dystrophy, characterized by muscle weakness, structural abnormalities in muscle fibers, and early onset of symptoms.
Full List of Targets in Fatty Acid Synthesis
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| ACACA | Acetyl-CoA Carboxylase Alpha; Acetyl-Coenzyme A Carboxylase Alpha; Acetyl-CoA Carboxylase 1; ACC-Alpha; ACAC; ACC1 | 31 | Q13085 | Acetyl-CoA carboxylase (ACC) is a complex multifunctional enzyme system. ACC is a biotin-containing enzyme which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting step in fatty acid synthesis. There are two ACC forms, alpha and beta, encoded by two different genes. ACC-alpha is highly enriched in lipogenic tissues. The enzyme is under long term control at the transcriptional and translational levels and under short term regulation by the phosphorylation/dephosphorylation of targeted serine residues and by allosteric transformation by citrate or palmitoyl-CoA. Multiple alternatively spliced transcript variants divergent in the 5' sequence and encoding distinct isoforms have been found for this gene. |
| ACACB | Acetyl-CoA Carboxylase Beta; Acetyl-Coenzyme A Carboxylase Beta; Acetyl-CoA Carboxylase 2; ACC-Beta; ACC2; ACCB; EC 6.4.1.2; HACC275 | 32 | O00763 | Acetyl-CoA carboxylase (ACC) is a complex multifunctional enzyme system. ACC is a biotin-containing enzyme which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting step in fatty acid synthesis. ACC-beta is thought to control fatty acid oxidation by means of the ability of malonyl-CoA to inhibit carnitine-palmitoyl-CoA transferase I, the rate-limiting step in fatty acid uptake and oxidation by mitochondria. ACC-beta may be involved in the regulation of fatty acid oxidation, rather than fatty acid biosynthesis. There is evidence for the presence of two ACC-beta isoforms. |
| ACLY | ATP Citrate Lyase; ATP-Citrate (Pro-S-)-Lyase; Citrate Cleavage Enzyme; EC 2.3.3.8; ACL; ATP Citrate Synthase; ATP-Citrate Synthase; ATPCL; CLATP; | 47 | A0A024R1T9 | ATP citrate lyase is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA in many tissues. The enzyme is a tetramer (relative molecular weight approximately 440,000) of apparently identical subunits. It catalyzes the formation of acetyl-CoA and oxaloacetate from citrate and CoA with a concomitant hydrolysis of ATP to ADP and phosphate. The product, acetyl-CoA, serves several important biosynthetic pathways, including lipogenesis and cholesterogenesis. In nervous tissue, ATP citrate-lyase may be involved in the biosynthesis of acetylcholine. Multiple transcript variants encoding distinct isoforms have been identified for this gene. |
| CHKB | CHKB; CHETK; Choline/Ethanolamine Kinase; CKEKB; EK; Choline/Ethanolamine Kinase Beta; EC 2.7.1.32; EKB; Choline Kinase-Like Protein; EC 2.7.1.82; CKB; CK; CHKL; MDCMC; Choline Kinase Beta | 1120 | Q9Y259 | Choline kinase (CK) and ethanolamine kinase (EK) catalyze the phosphorylation of choline/ethanolamine to phosphocholine/phosphoethanolamine. This is the first enzyme in the biosynthesis of phosphatidylcholine/phosphatidylethanolamine in all animal cells. The highly purified CKs from mammalian sources and their recombinant gene products have been shown to have EK activity also, indicating that both activities reside on the same protein. The choline kinase-like protein encoded by CHKL belongs to the choline/ethanolamine kinase family; however, its exact function is not known. Read-through transcripts are expressed from this locus that include exons from the downstream CPT1B locus. |
| FASN | FAS; OA-519; SDR27X1 | 2194 | P49327 | The enzyme encoded by this gene is a multifunctional protein. Its main function is to catalyze the synthesis of palmitate from acetyl-CoA and malonyl-CoA, in the presence of NADPH, into long-chain saturated fatty acids. In some cancer cell lines, this protein has been found to be fused with estrogen receptor-alpha (ER-alpha), in which the N-terminus of FAS is fused in-frame with the C-terminus of ER-alpha. |
| HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase; 3-Hydroxy-3-Methylglutaryl CoA Reductase (NADPH); 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase; Hydroxymethylglutaryl-CoA Reductase; HMG-CoA Reductase; EC 1.1.1.34; EC 1.1.1; LDLCQ3 | 3156 | P04035 | HMG-CoA reductase is the rate-limiting enzyme for cholesterol synthesis and is regulated via a negative feedback mechanism mediated by sterols and non-sterol metabolites derived from mevalonate, the product of the reaction catalyzed by reductase. Normally in mammalian cells this enzyme is suppressed by cholesterol derived from the internalization and degradation of low density lipoprotein (LDL) via the LDL receptor. Competitive inhibitors of the reductase induce the expression of LDL receptors in the liver, which in turn increases the catabolism of plasma LDL and lowers the plasma concentration of cholesterol, an important determinant of atherosclerosis. Alternatively spliced transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Aug 2008] |
| SOAT1 | SOAT1; ACACT1; Sterol O-Acyltransferase 1; ACACT; ACAT-1; Acyl-Coenzyme A:Cholesterol Acyltransferase 1; ACAT1; Cholesterol Acyltransferase 1; Sterol O-Acyltransferase (Acyl-Coenzyme A: Cholesterol Acyltransferase) 1; SOAT; STAT; ACAT | 6646 | P35610 | The protein encoded by this gene belongs to the acyltransferase family. It is located in the endoplasmic reticulum, and catalyzes the formation of fatty acid-cholesterol esters. This gene has been implicated in the formation of beta-amyloid and atherosclerotic plaques by controlling the equilibrium between free cholesterol and cytoplasmic cholesteryl esters. Alternatively spliced transcript variants have been found for this gene. |
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| ACACA | Acetyl-CoA Carboxylase Alpha; Acetyl-Coenzyme A Carboxylase Alpha; Acetyl-CoA Carboxylase 1; ACC-Alpha; ACAC; ACC1 | 31 | Q13085 | Acetyl-CoA carboxylase (ACC) is a complex multifunctional enzyme system. ACC is a biotin-containing enzyme which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting step in fatty acid synthesis. There are two ACC forms, alpha and beta, encoded by two different genes. ACC-alpha is highly enriched in lipogenic tissues. The enzyme is under long term control at the transcriptional and translational levels and under short term regulation by the phosphorylation/dephosphorylation of targeted serine residues and by allosteric transformation by citrate or palmitoyl-CoA. Multiple alternatively spliced transcript variants divergent in the 5' sequence and encoding distinct isoforms have been found for this gene. |
| ACACB | Acetyl-CoA Carboxylase Beta; Acetyl-Coenzyme A Carboxylase Beta; Acetyl-CoA Carboxylase 2; ACC-Beta; ACC2; ACCB; EC 6.4.1.2; HACC275 | 32 | O00763 | Acetyl-CoA carboxylase (ACC) is a complex multifunctional enzyme system. ACC is a biotin-containing enzyme which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting step in fatty acid synthesis. ACC-beta is thought to control fatty acid oxidation by means of the ability of malonyl-CoA to inhibit carnitine-palmitoyl-CoA transferase I, the rate-limiting step in fatty acid uptake and oxidation by mitochondria. ACC-beta may be involved in the regulation of fatty acid oxidation, rather than fatty acid biosynthesis. There is evidence for the presence of two ACC-beta isoforms. |
| ACLY | ATP Citrate Lyase; ATP-Citrate (Pro-S-)-Lyase; Citrate Cleavage Enzyme; EC 2.3.3.8; ACL; ATP Citrate Synthase; ATP-Citrate Synthase; ATPCL; CLATP; | 47 | A0A024R1T9 | ATP citrate lyase is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA in many tissues. The enzyme is a tetramer (relative molecular weight approximately 440,000) of apparently identical subunits. It catalyzes the formation of acetyl-CoA and oxaloacetate from citrate and CoA with a concomitant hydrolysis of ATP to ADP and phosphate. The product, acetyl-CoA, serves several important biosynthetic pathways, including lipogenesis and cholesterogenesis. In nervous tissue, ATP citrate-lyase may be involved in the biosynthesis of acetylcholine. Multiple transcript variants encoding distinct isoforms have been identified for this gene. |
| CHKB | CHKB; CHETK; Choline/Ethanolamine Kinase; CKEKB; EK; Choline/Ethanolamine Kinase Beta; EC 2.7.1.32; EKB; Choline Kinase-Like Protein; EC 2.7.1.82; CKB; CK; CHKL; MDCMC; Choline Kinase Beta | 1120 | Q9Y259 | Choline kinase (CK) and ethanolamine kinase (EK) catalyze the phosphorylation of choline/ethanolamine to phosphocholine/phosphoethanolamine. This is the first enzyme in the biosynthesis of phosphatidylcholine/phosphatidylethanolamine in all animal cells. The highly purified CKs from mammalian sources and their recombinant gene products have been shown to have EK activity also, indicating that both activities reside on the same protein. The choline kinase-like protein encoded by CHKL belongs to the choline/ethanolamine kinase family; however, its exact function is not known. Read-through transcripts are expressed from this locus that include exons from the downstream CPT1B locus. |
| FASN | FAS; OA-519; SDR27X1 | 2194 | P49327 | The enzyme encoded by this gene is a multifunctional protein. Its main function is to catalyze the synthesis of palmitate from acetyl-CoA and malonyl-CoA, in the presence of NADPH, into long-chain saturated fatty acids. In some cancer cell lines, this protein has been found to be fused with estrogen receptor-alpha (ER-alpha), in which the N-terminus of FAS is fused in-frame with the C-terminus of ER-alpha. |
| HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase; 3-Hydroxy-3-Methylglutaryl CoA Reductase (NADPH); 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase; Hydroxymethylglutaryl-CoA Reductase; HMG-CoA Reductase; EC 1.1.1.34; EC 1.1.1; LDLCQ3 | 3156 | P04035 | HMG-CoA reductase is the rate-limiting enzyme for cholesterol synthesis and is regulated via a negative feedback mechanism mediated by sterols and non-sterol metabolites derived from mevalonate, the product of the reaction catalyzed by reductase. Normally in mammalian cells this enzyme is suppressed by cholesterol derived from the internalization and degradation of low density lipoprotein (LDL) via the LDL receptor. Competitive inhibitors of the reductase induce the expression of LDL receptors in the liver, which in turn increases the catabolism of plasma LDL and lowers the plasma concentration of cholesterol, an important determinant of atherosclerosis. Alternatively spliced transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Aug 2008] |
| SOAT1 | SOAT1; ACACT1; Sterol O-Acyltransferase 1; ACACT; ACAT-1; Acyl-Coenzyme A:Cholesterol Acyltransferase 1; ACAT1; Cholesterol Acyltransferase 1; Sterol O-Acyltransferase (Acyl-Coenzyme A: Cholesterol Acyltransferase) 1; SOAT; STAT; ACAT | 6646 | P35610 | The protein encoded by this gene belongs to the acyltransferase family. It is located in the endoplasmic reticulum, and catalyzes the formation of fatty acid-cholesterol esters. This gene has been implicated in the formation of beta-amyloid and atherosclerotic plaques by controlling the equilibrium between free cholesterol and cytoplasmic cholesteryl esters. Alternatively spliced transcript variants have been found for this gene. |
Tested Data-Supported Products for Targeting Fatty Acid Synthesis
| CAT | Product Name | Biomarker | Assay | Image |
| ZG-0335J | Mouse Anti-FASN Recombinant Antibody (ZG-0335J) | FASN | WB |

|
| ZG-0336J | Mouse Anti-FASN Recombinant Antibody (ZG-0336J) | FASN | WB |

|
| ZG-0526J | Rabbit Anti-ACLY Recombinant Antibody (clone 3A5) | ACLY | WB |

|
| ZG-0665J | Rabbit Anti-FASN Recombinant Antibody (clone 2A5) | FASN | IF |

|
| VS3-FY16 | Recombinant Rabbit Anti-ACLY Antibody (clone R02-9B5) | ACLY | WB |

|
| VS3-FY686 | Recombinant Rabbit Anti-HMGCR Antibody (clone R04-2J3) | HMGCR | WB |

|
- Wallner, Stefan, et al. "Monocyte to macrophage differentiation goes along with modulation of the plasmalogen pattern through transcriptional regulation." PloS one 9.4 (2014): e94102.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
