

Medulloblastoma Biomarkers
Medulloblastoma represents a pivotal challenge in pediatric oncology, being the most common malignant brain tumor among children. Originating in the cerebellum, an area at the back of the skull that coordinates muscle movements and maintains posture and balance, this aggressive tumor accounts for approximately 20% of all pediatric brain tumors. The disease's complexity is underscored by its ability to metastasize or spread through the cerebrospinal fluid, a trait that complicates treatment and prognosis. Despite advances in medical science, the prognosis for medulloblastoma patients remains varied, heavily influenced by a constellation of factors including the patient's age at diagnosis, the presence and extent of metastasis, and the molecular and histological characteristics of the tumor. This variability renders a one-size-fits-all treatment approach ineffective, necessitating personalized treatment plans that can include surgery, radiation, and chemotherapy. The five-year survival rates for medulloblastoma patients are a testament to the disease's heterogeneity, ranging widely from 20% to over 100%, reflecting both the advances in treatment and the ongoing challenges in combating this formidable pediatric cancer.
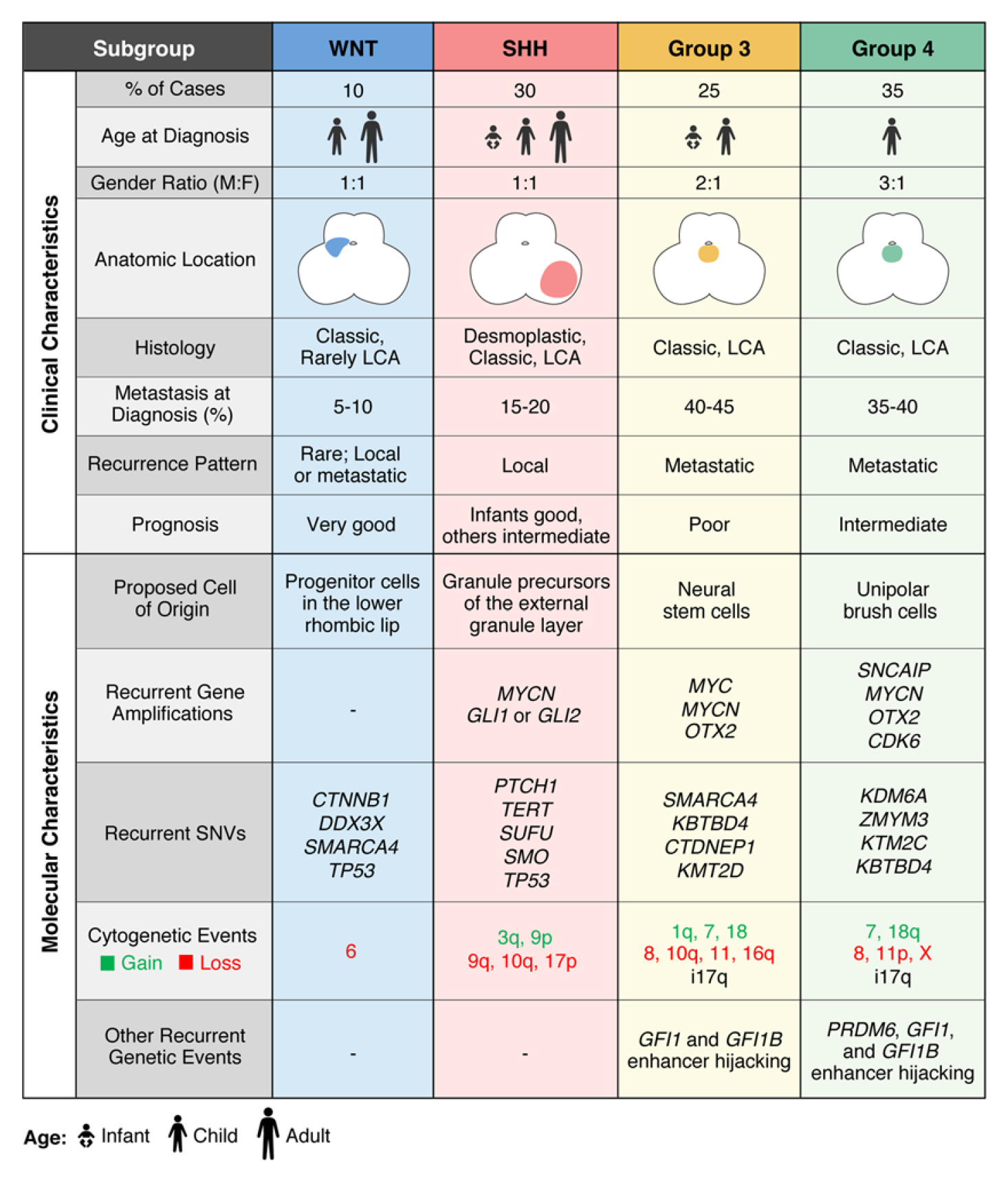 Figure 1 The molecular subgroups of medulloblastoma. (Juraschka, 2019)
Figure 1 The molecular subgroups of medulloblastoma. (Juraschka, 2019)
Representative Biomarkers of Medulloblastoma
VEGFA
VEGFA (Vascular Endothelial Growth Factor A) is a pivotal protein that plays a crucial role in angiogenesis, the process by which new blood vessels form from existing ones. It is essential for both physiological and pathological processes, including wound healing, the development of the embryonic vascular system, and the progression of diseases like cancer. In the context of medulloblastoma, VEGFA is of particular interest due to its role in tumor angiogenesis. Tumors require a blood supply to grow and metastasize, and VEGFA's ability to promote the formation of new blood vessels makes it a key player in medulloblastoma progression. High levels of VEGFA expression in medulloblastoma are associated with poor prognosis, increased tumor invasiveness, and resistance to therapy. Thus, targeting VEGFA and its signaling pathways has emerged as a promising therapeutic strategy in medulloblastoma treatment. By inhibiting VEGFA-mediated angiogenesis, it is possible to starve the tumor of its blood supply, thereby hindering its growth and spread. This focus on VEGFA not only underscores the complexity of tumor biology but also highlights the potential for innovative approaches to cancer therapy.


Tau
Tau is a microtubule-associated protein that plays a crucial role in stabilizing the cytoskeleton of neurons by binding to microtubules, which are essential for nutrient transport, cell signaling, and maintaining cell structure. In the healthy brain, Tau contributes to neuronal plasticity and integrity. However, its dysfunction is implicated in several neurodegenerative diseases, including Alzheimer's disease, where Tau proteins become hyperphosphorylated and form neurofibrillary tangles, leading to cell death. In the context of medulloblastoma, Tau's role is complex and emerging. Research suggests that Tau may participate in the pathogenesis and progression of medulloblastoma through its involvement in cell cycle regulation, apoptosis, and microtubule dynamics. Alterations in Tau expression or phosphorylation state could influence tumor cell proliferation, migration, and resistance to therapy. Although the exact mechanisms remain to be fully elucidated, understanding Tau's contribution to medulloblastoma biology could offer new avenues for targeted therapies, potentially improving outcomes for affected individuals.


LCN2
Lipocalin 2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL), is a secreted glycoprotein involved in various biological processes, including iron transport, inflammation, and cellular response to injury. In cancer biology, LCN2 has garnered attention for its role in tumor progression, metastasis, and response to therapy, particularly in medulloblastoma, the most common malignant pediatric brain tumor. Research has elucidated that LCN2 can influence the tumor microenvironment, modulating immune responses and affecting tumor cell proliferation and survival. Specifically, in medulloblastoma, LCN2 expression has been associated with tumor aggressiveness, poor prognosis, and resistance to conventional therapies. It is thought to contribute to oncogenesis through mechanisms such as promoting angiogenesis, facilitating tumor cell migration and invasion, and supporting stem cell-like characteristics in tumor cells. Thus, LCN2 not only serves as a potential biomarker for disease progression and prognosis in medulloblastoma but also presents a promising therapeutic target.


Full List of Medulloblastoma Biomarkers
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| APOA2 | apoAII; Apo-AII; ApoA-II | 336 | P02652 | This gene encodes apolipoprotein (apo-) A-II, which is the second most abundant protein of the high density lipoprotein particles. The protein is found in plasma as a monomer, homodimer, or heterodimer with apolipoprotein D. Defects in this gene may result in apolipoprotein A-II deficiency or hypercholesterolemia. |
| CLU | Clusterin; Testosterone-Repressed Prostate Message 2; Apolipoprotein J; Complement-Associated Protein SP-40,40; Complement Cytolysis Inhibitor; Complement Lysis Inhibitor; Sulfated Glycoprotein 2; Ku70-Binding Protein 1; NA1/NA2; TRPM-2; APO-J; APOJ; KUB1 | 1191 | P10909 | The protein encoded by this gene is a secreted chaperone that can under some stress conditions also be found in the cell cytosol. It has been suggested to be involved in several basic biological events such as cell death, tumor progression, and neurodegenerative disorders. Alternate splicing results in both coding and non-coding variants.[provided by RefSeq, May 2011] |
| FGF2 | Fibroblast Growth Factor 2; Fibroblast Growth Factor 2 (Basic); Heparin-Binding Growth Factor 2; HBGF-2; FGF-2; BFGF | 2247 | P09038 | The protein encoded by this gene is a member of the fibroblast growth factor (FGF) family. FGF family members bind heparin and possess broad mitogenic and angiogenic activities. This protein has been implicated in diverse biological processes, such as limb and nervous system development, wound healing, and tumor growth. The mRNA for this gene contains multiple polyadenylation sites, and is alternatively translated from non-AUG (CUG) and AUG initiation codons, resulting in five different isoforms with distinct properties. The CUG-initiated isoforms are localized in the nucleus and are responsible for the intracrine effect, whereas, the AUG-initiated form is mostly cytosolic and is responsible for the paracrine and autocrine effects of this FGF. [provided by RefSeq, Jul 2008] |
| IGFBP2 | Insulin Like Growth Factor Binding Protein 2 | 3485 | P18065 | The protein encoded by this gene is one of six similar proteins that bind insulin-like growth factors I and II (IGF-I and IGF-II). The encoded protein can be secreted into the bloodstream, where it binds IGF-I and IGF-II with high affinity, or it can remain intracellular, interacting with many different ligands. High expression levels of this protein promote the growth of several types of tumors and may be predictive of the chances of recovery of the patient. Several transcript variants, one encoding a secreted isoform and the others encoding nonsecreted isoforms, have been found for this gene. |
| IGFBP3 | IGFBP3; IBP3; IGF-Binding Protein 3; BP-53; IBP-3; IGFBP-3; Binding Protein 29; Binding Protein 53; Insulin-Like Growth Factor-Binding Protein 3; Growth Hormone-Dependent Binding Protein; Insulin-Like Growth Factor Binding Protein 3; Acid Stable Subunit O | 3486 | P17936 | This gene is a member of the insulin-like growth factor binding protein (IGFBP) family and encodes a protein with an IGFBP domain and a thyroglobulin type-I domain. The protein forms a ternary complex with insulin-like growth factor acid-labile subunit (IGFALS) and either insulin-like growth factor (IGF) I or II. In this form, it circulates in the plasma, prolonging the half-life of IGFs and altering their interaction with cell surface receptors. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. |
| IGFBP4 | Insulin Like Growth Factor Binding Protein 4 | 3487 | P22692 | This gene is a member of the insulin-like growth factor binding protein (IGFBP) family and encodes a protein with an IGFBP domain and a thyroglobulin type-I domain. The protein binds both insulin-like growth factors (IGFs) I and II and circulates in the plasma in both glycosylated and non-glycosylated forms. Binding of this protein prolongs the half-life of the IGFs and alters their interaction with cell surface receptors. |
| IGFBP6 | IGFBP6; insulin like growth factor binding protein 6; IBP6 | 3489 | P24592 | IGF-binding proteins prolong the half-life of the IGFs and have been shown to either inhibit or stimulate the growth promoting effects of the IGFs on cell culture. They alter the interaction of IGFs with their cell surface receptors. |
| LCN2 | Lipocalin 2; Oncogene 24p3; 25 KDa Alpha-2-Microglobulin-Related Subunit Of MMP-9; Neutrophil Gelatinase-Associated Lipocalin; Siderocalin LCN2; NGAL; P25; Migration-Stimulating Factor Inhibitor;Lipocalin 2 (Oncogene 24p3); Lipocalin-2; Siderocalin; 24p3; MSFI; HNL | 3934 | P80188 | This gene encodes a protein that belongs to the lipocalin family. Members of this family transport small hydrophobic molecules such as lipids, steroid hormones and retinoids. The protein encoded by this gene is a neutrophil gelatinase-associated lipocalin and plays a role in innate immunity by limiting bacterial growth as a result of sequestering iron-containing siderophores. The presence of this protein in blood and urine is an early biomarker of acute kidney injury. This protein is thought to be be involved in multiple cellular processes, including maintenance of skin homeostasis, and suppression of invasiveness and metastasis. Mice lacking this gene are more susceptible to bacterial infection than wild type mice. [provided by RefSeq, Sep 2015]LCN2 (Lipocalin 2) is a Protein Coding gene. Diseases associated with LCN2 include Klebsiella Infection and Kidney Disease. Among its related pathways are Defensins and Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds. Gene Ontology (GO) annotations related to this gene include protein homodimerization activity and iron ion binding. An important paralog of this gene is LCN12.Iron-trafficking protein involved in multiple processes such as apoptosis, innate immunity and renal development. Binds iron through association with 2,5-dihydroxybenzoic acid (2,5-DHBA), a siderophore that shares structural similarities with bacterial enterobactin, and delivers or removes iron from the cell, depending on the context. Iron-bound form (holo-24p3) is internalized following binding to the SLC22A17 (24p3R) receptor, leading to release of iron and subsequent increase of intracellular iron concentration. In contrast, association of the iron-free form (apo-24p3) with the SLC22A17 (24p3R) receptor is followed by association with an intracellular siderophore, iron chelation and iron transfer to the extracellular medium, thereby reducing intracellular iron concentration. Involved in apoptosis due to interleukin-3 (IL3) deprivation: iron-loaded form increases intracellular iron concentration without promoting apoptosis, while iron-free form decreases intracellular iron levels, inducing expression of the proapoptotic protein BCL2L11/BIM, resulting in apoptosis. Involved in innate immunity, possibly by sequestrating iron, leading to limit bacterial growth. |
| MMP2 | Matrix Metallopeptidase 2; Matrix Metalloproteinase-2; EC 3.4.24.24; CLG4A; MMP-2; TBE-1; Matrix Metalloproteinase 2 (Gelatinase A, 72kDa Gelatinase, 72kDa Type IV Collagenase); Matrix Metallopeptidase 2 (Gelatinase A, 72kDa Gelatinase, 72kDa Type IV Collagenase); Matrix Metalloproteinase-II; 72 KDa Type IV Collagenase | 4313 | P08253 | This gene is a member of the matrix metalloproteinase (MMP) gene family, that are zinc-dependent enzymes capable of cleaving components of the extracellular matrix and molecules involved in signal transduction. The protein encoded by this gene is a gelatinase A, type IV collagenase, that contains three fibronectin type II repeats in its catalytic site that allow binding of denatured type IV and V collagen and elastin. Unlike most MMP family members, activation of this protein can occur on the cell membrane. This enzyme can be activated extracellularly by proteases, or, intracellulary by its S-glutathiolation with no requirement for proteolytical removal of the pro-domain. This protein is thought to be involved in multiple pathways including roles in the nervous system, endometrial menstrual breakdown, regulation of vascularization, and metastasis. Mutations in this gene have been associated with Winchester syndrome and Nodulosis-Arthropathy-Osteolysis (NAO) syndrome. Alternative splicing results in multiple transcript variants encoding different isoforms. [provided by RefSeq, Oct 2014] |
| MMP9 | Matrix Metallopeptidase 9; Matrix Metalloproteinase 9 (Gelatinase B, 92kDa Gelatinase, 92kDa Type IV Collagenase); EC 3.4.24.35; CLG4B; MMP-9; GELB; Matrix Metallopeptidase 9 (Gelatinase B, 92kDa Gelatinase, 92kDa Type IV Collagenase); Matrix Metalloproteinase-9 | 4318 | P14780 | Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The enzyme encoded by this gene degrades type IV and V collagens. Studies in rhesus monkeys suggest that the enzyme is involved in IL-8-induced mobilization of hematopoietic progenitor cells from bone marrow, and murine studies suggest a role in tumor-associated tissue remodeling. [provided by RefSeq, Jul 2008] |
| NTN1 | NTN1; NTN1L; netrin 1 | 9423 | O95631 | Netrin is included in a family of laminin-related secreted proteins. The function of this gene has not yet been defined; however, netrin is thought to be involved in axon guidance and cell migration during development. Mutations and loss of expression of netrin suggest that variation in netrin may be involved in cancer development. |
| Tau | TAU; MSTD; PPND; DDPAC; MAPTL; MTBT1; MTBT2; tau-40; FTDP-17; PPP1R103; Tau-PHF6 | 4137 | P10636 | Immunotherapies designed to treat neurodegenerative tauopathies that primarily engage extracellular tau may have limited efficacy as tau is primarily intracellular. We generated tau-targeting single-chain variable fragments (scFvs) and intrabodies (iBs) from the phosphorylated tau-specific antibodies CP13 and PHF1 and the pan-tau antibody Tau5. Recombinant adeno-associated virus (rAAV) was utilized to express these antibody fragments in homozygous JNPL3 P301L tau mice. Two iBs (CP13i, PHF1i) and one scFv (PHF1s) abrogated tau pathology and delayed time to severe hindlimb paralysis. In a second tauopathy model (rTg4510), CP13i and PHF1i reduced tau pathology, but cognate scFvs did not. These data demonstrate that (1) disease-modifying efficacy does not require antibody effector functions, (2) the intracellular targeting of tau with phosphorylated tau-specific iBs is more effective than extracellular targeting with the scFvs, and (3) robust effects on tau pathology before neurodegeneration only resulted in modest disease modification as assessed by delay of severe motor phenotype. |
| VEGFA | MVCD1; VEGF; VPF | 7422 | P15692 | This gene is a member of the PDGF/VEGF growth factor family. It encodes a heparin-binding protein, which exists as a disulfide-linked homodimer. This growth factor induces proliferation and migration of vascular endothelial cells, and is essential for both physiological and pathological angiogenesis. Disruption of this gene in mice resulted in abnormal embryonic blood vessel formation. This gene is upregulated in many known tumors and its expression is correlated with tumor stage and progression. Elevated levels of this protein are found in patients with POEMS syndrome, also known as Crow-Fukase syndrome. Allelic variants of this gene have been associated with microvascular complications of diabetes 1 (MVCD1) and atherosclerosis. Alternatively spliced transcript variants encoding different isoforms have been described. There is also evidence for alternative translation initiation from upstream non-AUG (CUG) codons resulting in additional isoforms. A recent study showed that a C-terminally extended isoform is produced by use of an alternative in-frame translation termination codon via a stop codon readthrough mechanism, and that this isoform is antiangiogenic. Expression of some isoforms derived from the AUG start codon is regulated by a small upstream open reading frame, which is located within an internal ribosome entry site. |
Tested Data-Supported Products Targeting Medulloblastoma Biomarkers
- Juraschka, Kyle, and Michael D. Taylor. "Medulloblastoma in the age of molecular subgroups: a review: JNSPG 75th Anniversary Invited Review Article." Journal of Neurosurgery: Pediatrics 24.4 (2019): 353-363.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



-1-1.png)


-1.jpg)













