

Ovarian Cancer Biomarkers
Ovarian cancer, a formidable adversary in the realm of malignancies, demands our attention. It is a disease that affects the ovaries, those vital organs within the female reproductive system. More than 90% of ovarian cancers are believed to originate from epithelial cells, which either cover the ovarian surface or line subserosal cysts. Recent research has unveiled additional origins, including the fimbriae of Fallopian tubes and deposits of endometriosis. The disease manifests in distinct histotypes, resembling epithelial cells from various gynecological structures. Type I low-grade cancers present early, grow slowly, and exhibit resistance to conventional chemotherapy but may respond to hormonal manipulation. In contrast, type II high-grade cancers, diagnosed at advanced stages, grow aggressively but respond to chemotherapy. Understanding the molecular underpinnings, such as p53, BRCA1/2, and pathways like Ras/MAPK and PI3K, is crucial for advancing treatment strategies. Novel therapies targeting high-grade serous cancer cells and tumor vasculature are emerging, but the battle against ovarian cancer continues.
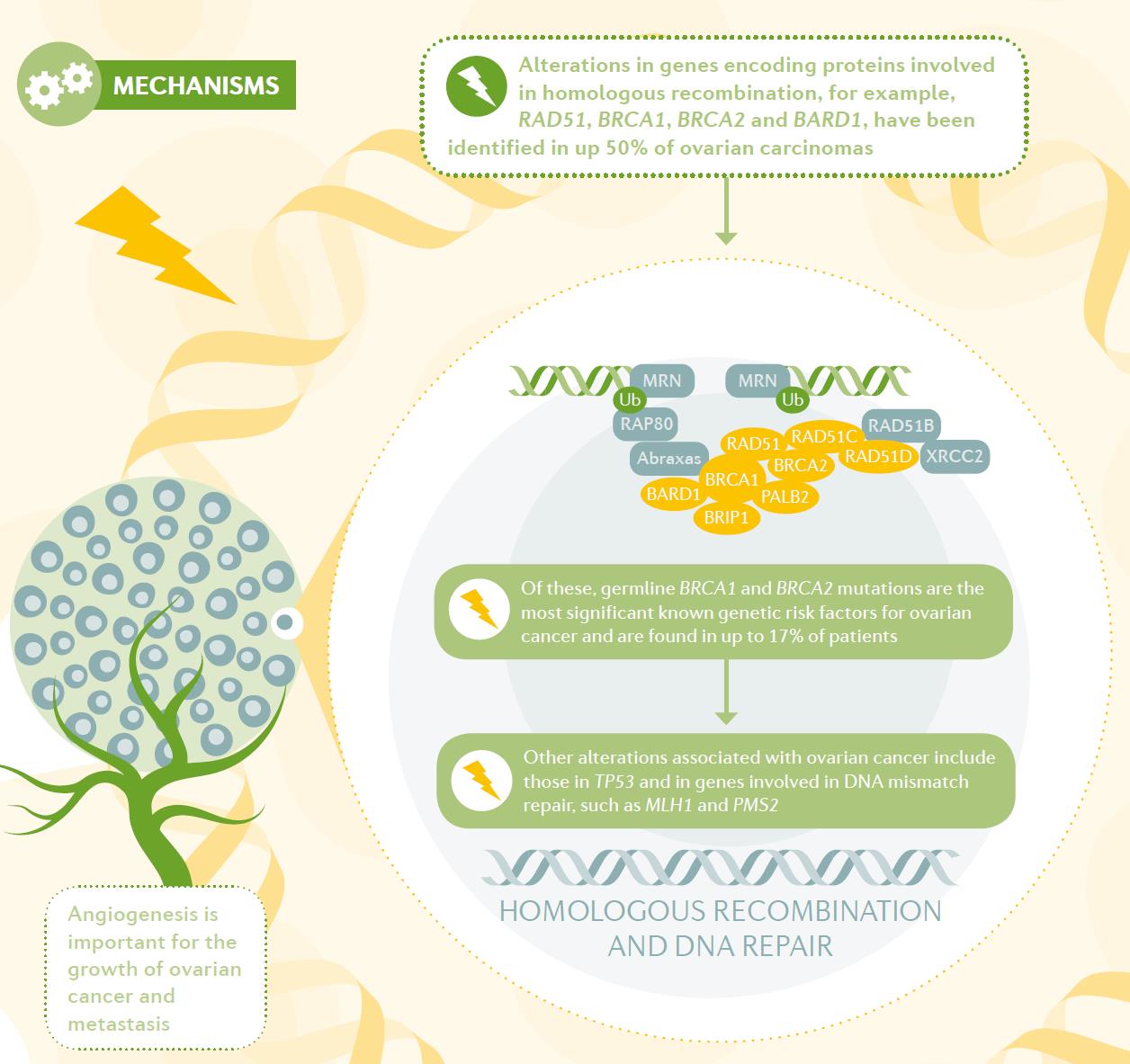 Figure 1 Mechanisms of ovarian cancer. (Matulonis, 2016)
Figure 1 Mechanisms of ovarian cancer. (Matulonis, 2016)
Representative Biomarkers of Ovarian Cancer
BRAF
The BRAF gene, an integral component of the mitogen-activated protein kinase (MAPK) signaling pathway, plays a pivotal role in regulating cell growth, proliferation, and differentiation. Mutations in the BRAF gene have been extensively studied in various cancers, including ovarian cancer, where they contribute to the dysregulation of cellular processes leading to oncogenesis. In ovarian cancer, BRAF mutations have been identified as key drivers of tumorigenesis, impacting disease progression and therapeutic outcomes. The most common mutation, BRAF V600E, results in constitutive activation of the MAPK pathway, leading to uncontrolled cell proliferation and evasion of apoptosis. Understanding the biology of BRAF in ovarian cancer is crucial for developing targeted therapies that specifically inhibit aberrant BRAF signaling, thereby offering potential avenues for personalized treatment strategies.



MSH2
MSH2, a pivotal gene encoding the DNA mismatch repair protein, plays a critical role in maintaining genomic stability and fidelity. Positioned within the context of DNA repair mechanisms, MSH2 functions as part of the MutS protein complex, orchestrating the identification and correction of mismatches that occur during DNA replication. Its significance extends beyond mere maintenance of genetic integrity, as MSH2's intricate involvement in cellular processes underpins the prevention of mutations and the suppression of tumorigenesis. However, disruptions in MSH2 function, whether through mutations or epigenetic modifications, can instigate a cascade of aberrant events, leading to genomic instability and predisposition to cancer development. In the context of ovarian cancer, MSH2's malfunction can have profound implications. Ovarian cancer, a complex and heterogeneous disease, often involves defects in DNA repair pathways, with deficiencies in mismatch repair being notably prevalent. Within this landscape, MSH2 emerges as a key player, with its dysregulation contributing to the accumulation of genetic errors and the progression of ovarian tumorigenesis.

MSH2
The phosphatase and tensin homolog (PTEN) gene serves as a vital regulator in various cellular processes, playing a pivotal role in the maintenance of cellular homeostasis and prevention of uncontrolled cell growth. Within the intricate landscape of cancer biology, PTEN emerges as a significant tumor suppressor gene, exerting its influence through its dual enzymatic activities as a lipid and protein phosphatase. Dysregulation or loss of PTEN function has been implicated in the development and progression of numerous cancer types, including ovarian cancer. In the context of ovarian cancer, PTEN alterations are prevalent, contributing to the aberrant activation of signaling pathways involved in cell proliferation, survival, and metastasis. Moreover, PTEN's involvement extends beyond its canonical tumor suppressive functions, influencing the response to various therapeutic modalities and dictating the clinical outcomes of ovarian cancer patients.
Full List of Ovarian Cancer Biomarkers
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| ASTL | Astacin Like Metalloendopeptidase; Astacin-Like Metallo-Endopeptidase (M12 Family); Sperm Acrosomal SLLP1 Binding; Oocyte Astacin; Ovastacin; Astacin-Like Metalloendopeptidase (M12 Family); Astacin-Like Metalloendopeptidase | 431705 | Q6HA08 | Oocyte-specific oolemmal receptor involved in sperm and egg adhesion and fertilization. Plays a role in the polyspermy inhibition. Probably acts as a protease for the post-fertilization cleavage of ZP2. Cleaves the sperm-binding ZP2 at the surface of the zona pellucida after fertilization and cortical granule exocytosis, rendering the zona pellucida unable to support further sperm binding. |
| ATAD2 | ATPase Family, AAA Domain Containing 2; AAA Nuclear Coregulator Cancer-Associated Protein; ANCCA; EC 3.6.1.3; PRO2000; CT137 | 29028 | A0A024R9G7 | A large family of ATPases has been described, whose key feature is that they share a conserved region of about 220 amino acids that contains an ATP-binding site. The proteins that belong to this family either contain one or two AAA (ATPases Associated with diverse cellular Activities) domains. AAA family proteins often perform chaperone-like functions that assist in the assembly, operation, or disassembly of protein complexes. The protein encoded by this gene contains two AAA domains, as well as a bromodomain. |
| BRAF | B-Raf Proto-Oncogene, Serine/Threonine Kinase; V-Raf Murine Sarcoma Viral Oncogene Homolog B1; V-Raf Murine Sarcoma Viral Oncogene Homolog B; Proto-Oncogene B-Raf; BRAF1; RAFB1; B-Raf Proto-Oncogene Serine/Threonine-Protein Kinase (P94); Murine Sarcoma Viral (V-Raf) Oncogene Homolog B1; Serine/Threonine-Protein Kinase B-Raf | 673 | P15056 | This gene encodes a protein belonging to the RAF family of serine/threonine protein kinases. This protein plays a role in regulating the MAP kinase/ERK signaling pathway, which affects cell division, differentiation, and secretion. Mutations in this gene, most commonly the V600E mutation, are the most frequently identified cancer-causing mutations in melanoma, and have been identified in various other cancers as well, including non-Hodgkin lymphoma, colorectal cancer, thyroid carcinoma, non-small cell lung carcinoma, hairy cell leukemia and adenocarcinoma of lung. Mutations in this gene are also associated with cardiofaciocutaneous, Noonan, and Costello syndromes, which exhibit overlapping phenotypes. A pseudogene of this gene has been identified on the X chromosome. |
| BRCA1 | IRIS; PSCP; BRCAI; BRCC1; FANCS; PNCA4; RNF53; BROVCA1; PPP1R53 | 672 | P38398 | This gene encodes a 190 kD nuclear phosphoprotein that plays a role in maintaining genomic stability, and it also acts as a tumor suppressor. The BRCA1 gene contains 22 exons spanning about 110 kb of DNA. The encoded protein combines with other tumor suppressors, DNA damage sensors, and signal transducers to form a large multi-subunit protein complex known as the BRCA1-associated genome surveillance complex (BASC). This gene product associates with RNA polymerase II, and through the C-terminal domain, also interacts with histone deacetylase complexes. This protein thus plays a role in transcription, DNA repair of double-stranded breaks, and recombination. Mutations in this gene are responsible for approximately 40% of inherited breast cancers and more than 80% of inherited breast and ovarian cancers. Alternative splicing plays a role in modulating the subcellular localization and physiological function of this gene. Many alternatively spliced transcript variants, some of which are disease-associated mutations, have been described for this gene, but the full-length natures of only some of these variants has been described. A related pseudogene, which is also located on chromosome 17, has been identified. |
| HER2 | NEU; NGL; HER2; TKR1; CD340; HER-2; MLN 19; HER-2/neu | 2064 | P04626 | This gene encodes a member of the epidermal growth factor (EGF) receptor family of receptor tyrosine kinases. This protein has no ligand binding domain of its own and therefore cannot bind growth factors. However, it does bind tightly to other ligand-bound EGF receptor family members to form a heterodimer, stabilizing ligand binding and enhancing kinase-mediated activation of downstream signalling pathways, such as those involving mitogen-activated protein kinase and phosphatidylinositol-3 kinase. Allelic variations at amino acid positions 654 and 655 of isoform a (positions 624 and 625 of isoform b) have been reported, with the most common allele, Ile654/Ile655, shown here. Amplification and/or overexpression of this gene has been reported in numerous cancers, including breast and ovarian tumors. Alternative splicing results in several additional transcript variants, some encoding different isoforms and others that have not been fully characterized. |
| KRAS | NS; NS3; CFC2; RALD; K-Ras; KRAS1; KRAS2; RASK2; KI-RAS; C-K-RAS; K-RAS2A; K-RAS2B; K-RAS4A; K-RAS4B; c-Ki-ras2 | 3845 | P01116 | This gene, a Kirsten ras oncogene homolog from the mammalian ras gene family, encodes a protein that is a member of the small GTPase superfamily. A single amino acid substitution is responsible for an activating mutation. The transforming protein that results is implicated in various malignancies, including lung adenocarcinoma, mucinous adenoma, ductal carcinoma of the pancreas and colorectal carcinoma. Alternative splicing leads to variants encoding two isoforms that differ in the C-terminal region. [provided by RefSeq, Jul 2008] |
| MSH2 | MutS Homolog 2; HMSH2; MutS (E. Coli) Homolog 2 (Colon Cancer, Nonpolyposis Type 1); MutS Homolog 2, Colon Cancer, Nonpolyposis Type 1 (E. Coli); MutS Homolog 2, Colon Cancer, Nonpolyposis Type 1; DNA Mismatch Repair Protein Msh2; MutS Protein Homolog 2 | 4436 | P43246 | This locus is frequently mutated in hereditary nonpolyposis colon cancer (HNPCC). When cloned, it was discovered to be a human homolog of the E. Coli mismatch repair gene mutS, consistent with the characteristic alterations in microsatellite sequences (RER+ phenotype) found in HNPCC. Two transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Apr 2012] |
| NAPSA | Napsin A Aspartic Peptidase; Kidney-Derived Aspartic Protease-Like Protein; Aspartyl Protease 4; TA01/TA02; Napsin-1; Asp 4; NAP1; ASP4; NAPA; EC 3.4.23.15 | 9476 | O96009 | This gene encodes a member of the peptidase A1 family of aspartic proteases. The encoded preproprotein is proteolytically processed to generate an activation peptide and the mature protease. The activation peptides of aspartic proteinases function as inhibitors of the protease active site. These peptide segments, or pro-parts, are deemed important for correct folding, targeting, and control of the activation of aspartic proteinase zymogens. The encoded protease may play a role in the proteolytic processing of pulmonary surfactant protein B in the lung and may function in protein catabolism in the renal proximal tubules. This gene has been described as a marker for lung adenocarcinoma and renal cell carcinoma. [provided by RefSeq, Feb 2016] |
| p53 | 7157 | K7PPA8 | ||
| PTEN | BZS; DEC; CWS1; GLM2; MHAM; TEP1; MMAC1; PTEN1; 10q23del; PTENbeta | 5728 | P60484 | This gene was identified as a tumor suppressor that is mutated in a large number of cancers at high frequency. The protein encoded by this gene is a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase. |
Tested Data-Supported Products Targeting Ovarian Cancer Biomarkers
- Matulonis, Ursula A et al. “Ovarian cancer.” Nature reviews. Disease primers vol. 2 16061. 25 Aug. 2016.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.















