

Mitochondrial Metabolism
Mitochondrial metabolism encompasses a suite of biochemical processes that occur within the mitochondria, the powerhouse of the cell, which are crucial for energy production, signaling, and cellular homeostasis. This multifaceted system includes the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, fatty acid oxidation, and the handling of reactive oxygen species (ROS), among other processes. Collectively, these pathways not only produce ATP, the primary energy currency of the cell, but also generate metabolic intermediates that serve as building blocks for the synthesis of essential cellular components. The TCA cycle, also known as the Krebs cycle, lies at the heart of mitochondrial metabolism. It oxidizes acetyl-CoA derived from carbohydrates, fats, and proteins into carbon dioxide, and in the process, transfers energy to electron carriers NADH and FADH2. These carriers then donate electrons to the electron transport chain (ETC) in the inner mitochondrial membrane, driving the production of ATP through a process known as oxidative phosphorylation. This stage is highly efficient and produces the majority of ATP in aerobic organisms. Mitochondrial metabolism also plays a key role in the regulation of cellular energy homeostasis. It adapts to changes in energy demand by altering the rates of oxidative phosphorylation and other metabolic pathways, a regulation mediated by signaling molecules such as AMPK and sirtuins, which sense the energy status of the cell and adjust mitochondrial activity accordingly. Moreover, mitochondria are pivotal in managing cellular calcium levels, synthesizing critical hormones and other molecules, and initiating apoptotic pathways that are essential for the removal of damaged cells, thereby preventing pathological conditions. Dysfunctions in mitochondrial metabolism are linked to a wide array of diseases, including metabolic disorders like diabetes, neurodegenerative diseases such as Parkinson’s and Alzheimer's, and various forms of cancer.
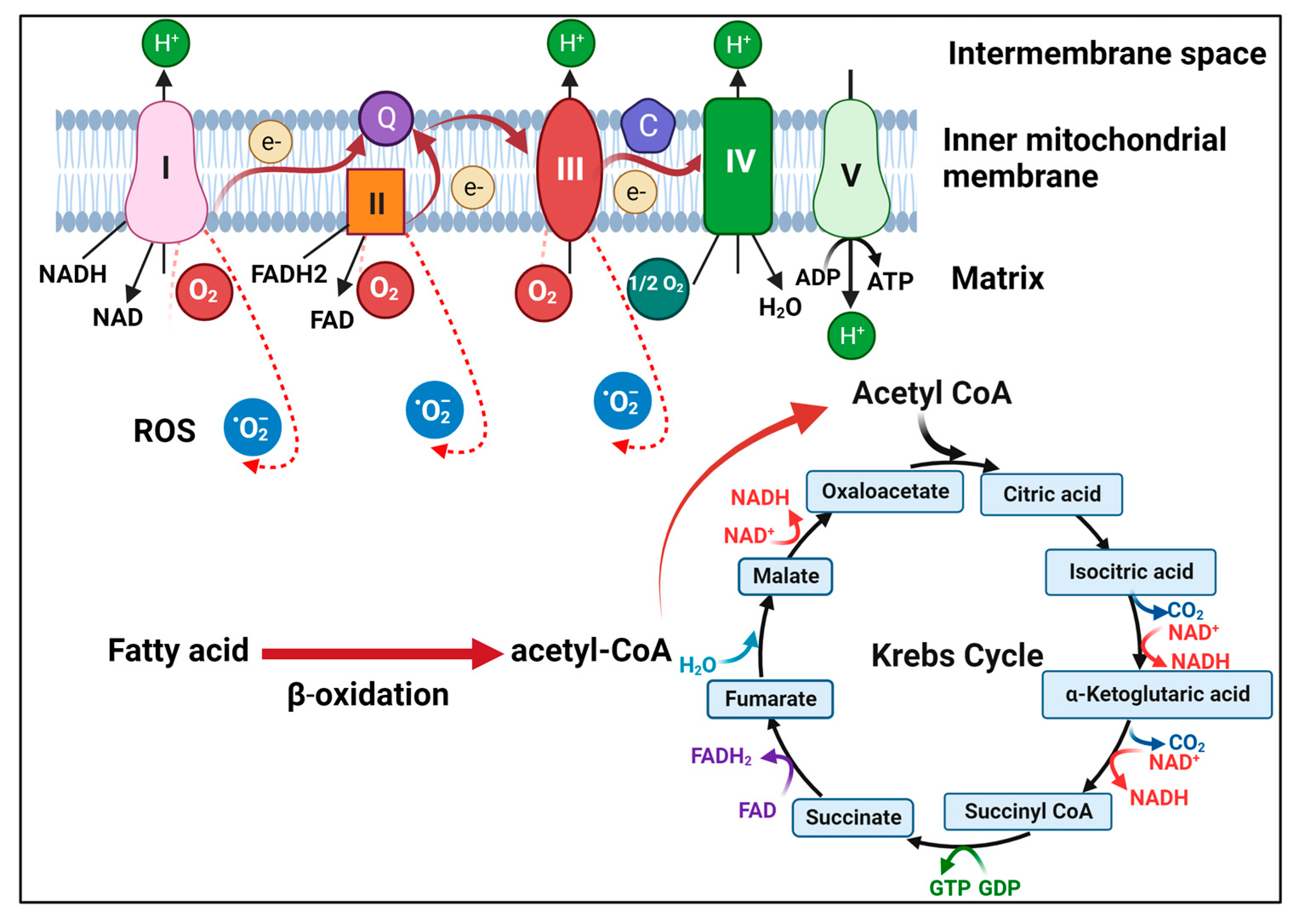 Figure 1 Illustration of mitochondrial metabolism. (Li, 2023)
Figure 1 Illustration of mitochondrial metabolism. (Li, 2023)
Representative Targets in Mitochondrial Metabolism
HIF1A
HIF1A, or Hypoxia-Inducible Factor 1 Alpha, is a transcription factor that plays a role in cellular response to oxygen availability. Under normal oxygen conditions (normoxia), HIF1A is rapidly degraded. However, under low oxygen conditions (hypoxia), it stabilizes, accumulates, and translocates to the nucleus where it dimerizes with HIF1 beta (also known as ARNT) to form the active HIF-1 complex. This complex binds to hypoxia-responsive elements (HREs) in the DNA and activates the transcription of numerous genes involved in various adaptive responses to hypoxia. These genes regulate processes critical for survival under low oxygen conditions, including angiogenesis (the formation of new blood vessels), erythropoiesis (production of red blood cells), glucose metabolism (enhancing glycolysis and reducing dependence on oxygen-consuming processes), and cell survival. The role of HIF1A is not limited to hypoxic response; it also has implications in various physiological and pathological processes, including development, wound healing, and tumor progression. In cancer, HIF1A is particularly noteworthy for its role in tumor microenvironments, where it often becomes constitutively active due to intra-tumoral hypoxia or genetic alterations. This activation promotes the survival of cancer cells in adverse conditions by facilitating metabolic reprogramming, enhancing angiogenesis, and promoting invasion and metastasis.

SOD1
SOD1, or Superoxide Dismutase 1, is a enzyme that plays a vital role in cellular defense against oxidative stress. It is primarily responsible for catalyzing the dismutation of the superoxide radical (O2-) into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2). SOD1 is a ubiquitous enzyme found in almost all cellular compartments, but notably in the cytoplasm, where it forms an important part of the antioxidant defense system, protecting cells from damage induced by reactive oxygen species (ROS). The antioxidant activity of SOD1 is crucial for maintaining cellular homeostasis and preventing oxidative stress-related damage to cellular components such as proteins, lipids, and DNA. Dysregulation or mutations in the SOD1 gene can lead to the accumulation of superoxide radicals, resulting in increased oxidative stress and cellular damage. This has been linked to various diseases, particularly neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease. Mutations in SOD1 are one of the known genetic causes of familial ALS, where they are believed to result in a toxic gain of function rather than a loss of dismutase activity. In ALS, the mutated SOD1 protein is thought to form aggregates that disrupt cellular function, including mitochondrial dysfunction, excitotoxicity, and impairment of axonal transport, contributing to the degeneration of motor neurons. The exact mechanisms by which SOD1 mutations lead to motor neuron death are still under intensive study, with the aim of developing targeted therapies that can mitigate this effect.

GSR
GSR, or Glutathione Reductase, is a enzyme in the antioxidant defense system of cells, primarily responsible for maintaining the reduced state of glutathione (GSH), a key antioxidant. Glutathione reductase catalyzes the reduction of glutathione disulfide (GSSG) back to its sulfhydryl form (GSH) using NADPH as a reducing agent. This reaction is essential for the regeneration of glutathione, enabling it to continue its role in detoxifying reactive oxygen species (ROS) such as hydrogen peroxide and lipid peroxides. The enzyme GSR is ubiquitously present in both prokaryotic and eukaryotic cells, highlighting its fundamental importance across various life forms. In humans, it is particularly critical in red blood cells where it helps protect against oxidative damage caused by ROS, which can lead to hemolysis and anemia if unchecked. Moreover, GSR's activity is crucial in other tissues that are frequently exposed to oxidative stress, such as the liver, kidney, and lungs. Dysfunction or deficiency in glutathione reductase can lead to a decrease in the ratio of reduced to oxidized glutathione within cells, thereby impairing the cellular antioxidant capacity and increasing vulnerability to oxidative stress. This has implications for various clinical conditions, including cardiovascular diseases, neurodegenerative disorders, diabetes, and cancer, where oxidative stress plays a pathogenic role.

Full List of Targets in Mitochondrial Metabolism
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| PTK2 | PTK2; FAK; FAK1; Focal adhesion kinase 1; FADK 1; Focal adhesion kinase-related nonkinase; FRNK; Protein phosphatase 1 regulatory subunit 71; PPP1R71; Protein-tyrosine kinase 2; p125FAK; pp125FAK | 5747 | Q05397 | PTK2 protein tyrosine kinase 2 (PTK2), also known as focal adhesion kinase (FAK), is a protein that, in humans, is encoded by the PTK2 gene. |
| SRC | SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase; V-Src Avian Sarcoma (Schmidt-Ruppin A-2) Viral Oncogene Homolog; Proto-Oncogene C-Src; EC 2.7.10.2; P60-Src; SRC1; Proto-Oncogene Tyrosine-Protein Kinase Src; Protooncogene SRC, Rous Sarcoma | 396442 | P00523 | This gene is highly similar to the v-src gene of Rous sarcoma virus. This proto-oncogene may play a role in the regulation of embryonic development and cell growth. The protein encoded by this gene is a tyrosine-protein kinase whose activity can be inhibited by phosphorylation by c-SRC kinase. Mutations in this gene could be involved in the malignant progression of colon cancer. Two transcript variants encoding the same protein have been found for this gene. [provided by RefSeq, Jul 2008] |
Tested Data-Supported Products for Targeting Mitochondrial Metabolism
| CAT | Product Name | Biomarker | Assay | Image |
| ZG-0442F | Mouse Anti-Src Recombinant Antibody (ZG-0442F) | Src | WB |

|
| ZG-0443F | Mouse Anti-Src Recombinant Antibody (ZG-0443F) | Src | WB |

|
| ZG-0329J | Mouse Anti-PTK2 Recombinant Antibody (ZG-0329J) | PTK2 | WB |

|
| ZG-0330J | Mouse Anti-PTK2 Recombinant Antibody (ZG-0330J) | PTK2 | WB |

|
| ZG-0331J | Mouse Anti-PTK2 Recombinant Antibody (ZG-0331J) | PTK2 | WB |

|
| ZG-0332J | Mouse Anti-PTK2 Recombinant Antibody (ZG-0332J) | PTK2 | WB |

|
| ZG-0100U | Rabbit Anti-PTK2 Recombinant Antibody (clone 3B2) | PTK2 | IHC |

|
| ZG-0531U | Rabbit Anti-Phospho-PTK2 (Y397) Recombinant Antibody (clone 1B3) | PTK2 | WB |

|
| ZG-0577U | Rabbit Anti-SRC Recombinant Antibody (clone 21H5) | SRC | WB |

|
| VS3-FY1221 | Recombinant Rabbit Anti-PTK2 Antibody (clone R08-3C1) | PTK2 | WB |

|
| VS3-FY1388 | Recombinant Rabbit Anti-SRC (phospho Tyr529) Antibody (clone R03-8H8) | SRC | WB |

|
| VS3-FY1389 | Recombinant Rabbit Anti-SRC Antibody (clone R05-7H8) | SRC | WB |

|
- Li, Yumei, and Jing-hsiung James Ou. "Regulation of Mitochondrial Metabolism by Hepatitis B Virus." Viruses 15.12 (2023): 2359.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
