

Statistical Equivalence Analysis
What Is Equivalence Analysis?
Biosimilars are safe, affordable and effective treatment alternatives as almost an identical copy of its originator. Because of unavoidable differences in the manufacturing processes, a biosimilar is not likely to be entirely identical to the reference product. An equivalence trial should show that the proposed biosimilar has neither decreased nor increased activity relative to the reference product, in other words, neither the biosimilar nor the reference product is superior or inferior to the other.
To evaluate the equivalence of a biosimilar to its reference product, many regulatory authorities have published access requirements for approving biosimilars. As a leading provider of the development and manufacturing of recombinant antibodies, Creative Biolabs have a well-established antibody platform, with extensive successful development cases. Now we expand our knowledge into antibody biosimilar areas. Our experts have the insight needed in biosimilar development, regulatory conditions, and commercialization. We have a reliable statistical analysis to demonstrate equivalence of biosimilars to the reference drugs.
Equivalence Analysis in Biosimilar Development
The major focus when designing an equivalence trial is to determine equivalence margins and sample size. The equivalence margin presents the largest difference that can be judged as being clinically acceptable between the similar and the reference product. Typically the two one-sided t-test (TOST) is used to demonstrate equivalence. If the confidence interval is within the equivalence margin then biosimilar and reference products are equivalent. The value of quivalence margins in equivalence trials conventionally varies from 0.3% to 15%.
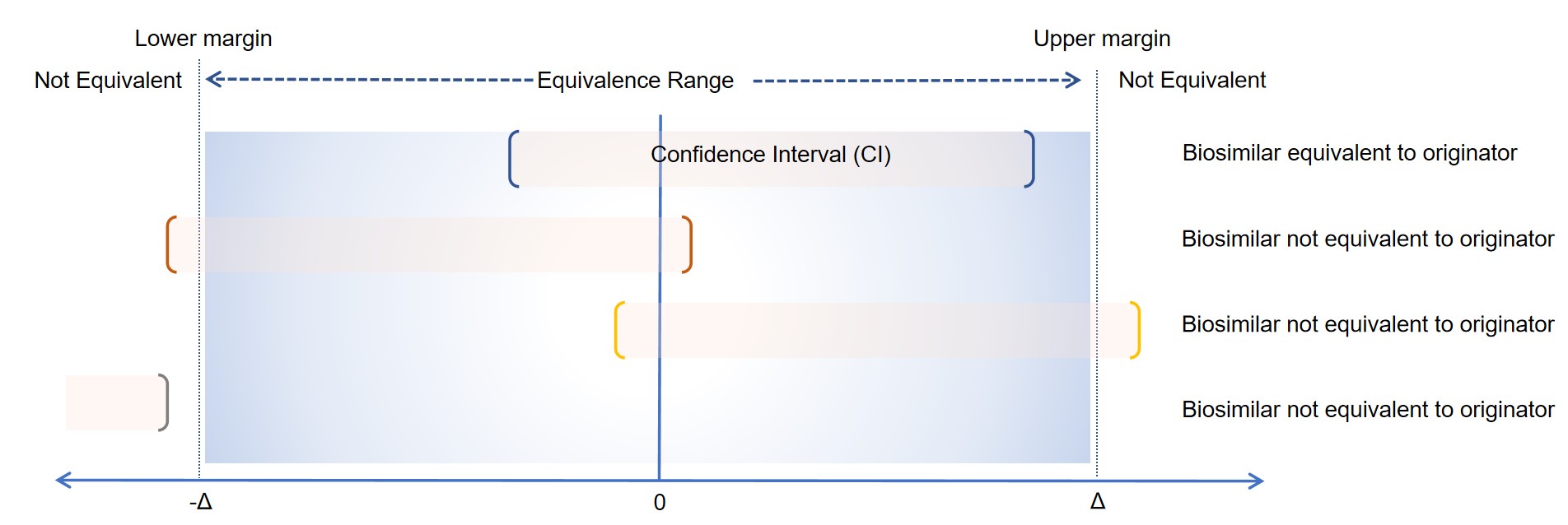 Fig.1 Examples of statistical equivalence testing outcomes. (Creative Biolabs)
Fig.1 Examples of statistical equivalence testing outcomes. (Creative Biolabs)
Creative Biolabs designs robust and rigorous statistical equivalence analysis on each biosimilar project. Our statisticians specialize in statistical analyses, ensuring compliant data to cope with complex regulatory requirements. Our statisticians have experience in tackling statistical issues and challenges in the development of biosimilars, including:
- Selection of appropriate and sensitive endpoints
- Determination of margins and sample size
- Quantification of totality-of-the-evidence
- Statistical considerations
Find Out More Stages in Biosimilar Development
- Clinical studies (Safety, efficacy, immunogenicity)
- Clinical pharmacology (PK/PD)
- In vivo studies (non-clinical)
- In vitro studies (analytical characterization)
 Biosimilar Development
Biosimilar Development
- Human studies: to evaluate similarity
- Human studies: PK/PD
- In vivo non-clinical pharmacokinetics/ pharmacodynamics analysis, toxicokinetics analysis
- Physicochemical, biological activity, stability characterization
Our Development Area in Biosimilars
We are bringing our expertise to biosimilar development programs including recombiniant antibody and protein biosimilars.

Collaboration
Analytical equivalence is the foundation for the demonstration of biosimilarity between a biosimilar and a reference product. At Creative Biolabs, each staff of our statistician team is familiar with and has expertise in equivalence testing. We develop an effective plan and execution strategy for the equivalence study. If you need a partner with proven experience in biosimilar development, please feel free to discuss partnerships with us.
Reference
- de Oliveira Ascef, B.; et al. Therapeutic Equivalence of biosimilar and reference biologic drugs in rheumatoid arthritis: a systematic review and meta-analysis. JAMA Network Open. 2023, 6(5): e2315872-e2315872.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
