Sifalimumab Overview
Introduction of Sifalimumab
Sifalimumab is a fully human IgG1 type monoclonal antibody directed against interferon α (IFN-α), which is a member of interferon type I. This drug has been investigated in the treatment of trails studying systemic lupus erythematosus (SLE), dermatomyositis, and polymyositis.
Most of the trials on sifalimumab have been done on systemic lupus erythematosus. Earlier in 2011, the phase I trial to assess the safety profile, pharmacokinetics, immunogenicity, pharmacodynamics and clinical activity of sifalimumab had conducted. Results showed that sifalimumab had a safety profile that supports further clinical development and IFNα inhibition may be associated with clinical benefit in systemic lupus erythematosus. Further, in 2013, the safety and tolerability of multiple intravenous (IV) doses of sifalimumab in adults with moderate-to-severe systemic lupus erythematosus had carried out, observing that safety/tolerability and clinical activity profile of sifalimumab support its continued clinical development for systemic lupus erythematosus. Then, a pharmacokinetic study of sifalimumab in systemic lupus erythematosus had assessed to find reasonable dosing regimens. The phase IIb trial of the pharmacokinetics of sifalimumab conducted to evaluate the utility of fixed dosing in 2016. In addition, sifalimumab had investigated in the trial studying dermatomyositis and polymyositis. The study assessed the pharmacodynamics effects of sifalimumab in the blood and muscle of adult dermatomyositis and polymyositis patients by measuring neutralization of a type I IFN gene signature (IFNGS) following drug exposure in 2014. Although the result was in a positive correlative trend between target neutralization and clinical improvement, more studies are required to confirm its efficacy.
Mechanism of Action of Sifalimumab
Sifalimumab is designed to bind to most of the IFN-α subtype with high affinities. IFN-α is a type I interferon factor which is essential to a vast array of immunologic processes. Type I interferons consist of a large number of interferon proteins that defense against viral infections and help regulate the activity of the immune system. By binding to type I IFN receptor (IFNAR), type I interferons trigger several signal transduction pathways and play important roles in multiple pathological and physiological processes. The receptor of all type I interferons is a specific cell surface protein complex known as the IFN-α receptor (IFNAR) which is composed by IFNAR1 and IFNAR2 chains. The downstream signal related to type I interferons are Janus Kinase/signal transducer and activator of transcription (JAK/STAT) pathway which are mediated by Janus Kinase 1 (JAK1) and tyrosine kinase 2 (TYK2). Type I interferons have been demonstrated to involve in diseases associated with chronic infection, autoimmunity and virus infection. IFN-α is one of the eight cytokines of type I interferon subfamily. It is contributed to orchestrate subsequent signal pathways in innate and adaptive immunity. Disease associated with IFN-α including autoimmune thyroid disease, systemic lupus erythematosus, rheumatoid arthritis, primary biliary cholangitis, and type 1 diabetes.
Sifalimumab is a monoclonal antibody drug targeted at IFN-α to block the interferon signaling. This drug is an approximately 147 kDa protein composed of two identical heavy chains and two identical light chains. Mechanism of action of sifalimumab works in systemic lupus erythematosus might through suppress the abnormal immune activity associated with lupus by binding to multiple interferon-alpha subtypes seen in the serum of lupus patients.
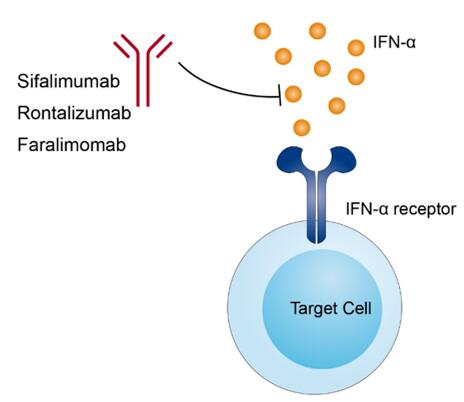 Fig.1 Mechanism of action of Sifalimumab
Fig.1 Mechanism of action of Sifalimumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



