Why Recombinant Monoclonal Antibodies?
Antibodies are groups of globular proteins of serum/plasma, which are also known as immunoglobulins. Antibodies "react" specifically with antigens that trigger the generation of those specific antibodies. The "antibody" and "immunoglobulin" are used interchangeably in the literature. Immunoglobulin can represent any antibody-like molecule regardless of its antigen-binding specificity.
Antibodies, considered as the workforce for a humoral response, perform two major functions: (1) target recognition via the Fab portion and (2) antigen elimination/effector function via the Fc portion. The ultimate aim of antibody response is to neutralize or eliminate any threat encountered by the immune system. Antibodies are part of the adaptive immune response, which, therefore, is specific against the threat/infection; specific antibodies bind to antigens with high affinity. These two characteristics make antibodies ideal tools for a variety of purposes in scientific and medical research.
Antibodies are widely used in disease diagnosis and treatment and are one of the most valuable research objects. The antibody preparation technology has gone through three stages. In the first stage, the antibodies are purified from the serums of the animal immunized with antigens. The antibodies obtained are polyclonal. In the second stage, the hybridoma technology came out. Tumor cells are fused with antibody-producing B lymphocytes to produce monoclonal antibodies against a single antigenic determinant. In the third stage, the gene sequence of monoclonal antibodies produced by animals is modified through genetic engineering technology to make the performance of monoclonal antibodies more in line with research needs. And the antibodies can be obtained by large-scale cell culture, which, at this stage, are recombinant antibodies.
What Are Monoclonal Antibodies? Monoclonal Antibodies V.S. Polyclonal Antibodies What Are Recombinant Antibodies? Categories of Recombinant Antibodies Production of Recombinant AntibodiesRecombinant Antibody BenefitsAdvantages of Creative Biolabs' Hi-Affi™ Recombinant AntibodyCustomer Testimonials
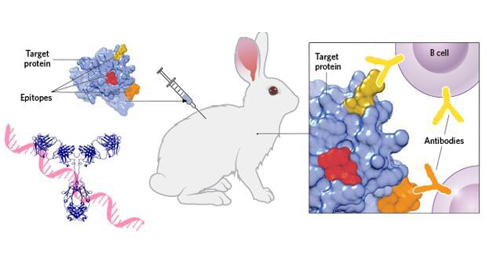
What Are Monoclonal Antibodies?
Monoclonal antibodies (mAbs) are highly homogeneous antibodies produced by a single B cell clone directed against a certain specific epitope. mAbs are usually prepared by hybridoma technology that is based on cell fusion - by fusing sensitized B cells with the ability to secrete specific antibodies and myeloma cells with unlimited reproductive ability into hybridoma cells. A single hybridoma cell is used to grow a cell population to produce a specific antibody against an antigenic epitope, that is, a monoclonal antibody.
Monoclonal antibodies are the dominant group of recombinant proteins used in human therapy. These proteins were first successfully developed by Köhler and Milstein in the mid-1970s and published in 1975 free of intellectual property (IP) rights, who produced a method for B-cell immortalization and the production of monoclonal antibodies. They fused mouse myeloma cells that can be cultured and proliferated in vitro with pure mouse B cells immunized with antigen to form a hybrid cell line, which not only has the characteristics of tumor cells being easy to proliferate indefinitely in vitro but also can form antibodies. The hybridoma can be cultured as a single cell to form a single cell line, that is, a single clone. Using the method of culture or intraperitoneal inoculation of mice, a large number of high-concentration and very uniform antibodies can be obtained, whose structure, amino acid sequence, specificity, etc. are all the same. Antibodies secreted over time maintain the same structure and function. The mAbs have various applications in cell biology, immunology, biotechnology, and medicines. They are also being used as in vivo imaging techniques for different diseases.
Monoclonal Antibodies V.S. Polyclonal Antibodies
Monoclonal antibodies and polyclonal antibodies are terms commonly found in the immunology literature. Both antibodies have the same structure and functions but differ from each other in terms of their origin, production, and specificity. The fundamental difference between these two kinds lies in the clonality of the cells producing them. Monoclonal antibodies are produced by a single clone, while polyclonal antibodies are by multiple clones together. Each of these antibodies has its pros and cons, shown as follows.
| Polyclonal Antibody | Monoclonal Antibody | |
| Characteristics | ||
| Adverse Effect | Serum sickness | Human anti-monoclonal antibody |
| Affinity Purified Product | Not homogenous | Homogenous |
| Antibody Purification | May/May not | Not essential (depends on application) |
| Booster Dose | Required | Not required |
| Chemically Defined | Not well | Well |
| Clonality | Numerous | Single |
| Cross-reactivity | High to low | Low to nil |
| Degradability | Low | High |
| Denatured Antigen (Detection of antigen) | Detect | May/May not |
| Epitope Detection | Multiple | Single |
| Homogeneity (Antibody) | No | Yes |
| Manpower Skills | Low skill | Highly trained |
| Origin | Any | Mouse (mostly) |
| Production Cost | ||
| Initial | Low | High |
| Long-Term | High | Low |
| Production Requirement | ||
| In vitro | - | + |
| In vivo | + | + |
| Specificity | Low | High |
| Variability (Lot-to-Lot) | High | Low |
What Are Recombinant Antibodies?
After the advent of monoclonal antibody technology, mouse-derived monoclonal antibodies have been applied to clinical treatment, which, however, are xenogeneic antigens to humans, and repeated injections can cause humans to produce anti-mouse antibodies, thereby weakening or losing their efficacy. Therefore, in the early 1980s, people began to use genetic engineering technology to prepare antibodies to reduce the immunogenicity of murine antibodies. These genetically engineered antibodies are termed recombinant antibodies, referring to antibody molecules expressed by transfecting appropriate recipient cells after processing and reassembling the gene encoding the antibody using recombinant DNA and protein engineering techniques according to different needs.
Recombinant antibodies and monoclonal antibodies are two different dimensions, although recombinant antibodies for therapeutic use are usually monoclonal. The recombinant antibodies targeting the same antigens or epitopes can be considered monoclonal antibodies, whereas recombinant antibodies with different specificities are polyclonal antibodies.
Categories of Recombinant Antibodies
Recombinant antibodies can be divided into five main categories: chimeric antibodies, humanized antibodies, fully human antibodies, small molecule antibodies, and bispecific antibodies.
The constant and variable regions of antibodies derive from different species, and common chimeric antibodies combine the variable regions of animal-derived antibodies with the constant regions of human-derived antibodies. The variable region, an animal-derived region, retains the specificity and affinity of the antibody to the antigen. Nearly 70% of the antibody is human, greatly reducing the heterogeneity of the antibody. The human Fc fragment can effectively mediate ADCC (antibody-dependent cell-mediated cytotoxicity) and CDC (complement-dependent cytotoxicity) effects. For the design and production of chimeric antibodies, different antibody subtypes, sizes, modification sites, etc., can be selected as needed. Through mature plasmid systems and protein expression platforms, the target chimeric antibodies can be obtained efficiently and in large quantities.
Replacing the complementarity-determining regions (CDRs) of human antibodies with CDRs of animal-derived monoclonal antibodies generates the humanized antibodies, also known as CDR-grafted antibodies.
Humanized antibodies further expand the humanized region of antibodies based on the chimeric antibodies, and the proportion of humanization can reach 80%-90%, so that the antibodies can reduce the heterologous rejection of the human body during in vivo application process. The binding process of CDR and antigen is affected by the framework region (FR). The combination of animal-derived CDR and human-derived FR may change the spatial structure of the original CDR of the antibody, thereby reducing the affinity of the recombinant antibody to the antigen. When designing humanized antibodies, key amino acid residues in the human FR can be replaced with animal-derived FRs to reduce the impact on the CDR domains.
With gene knock-out technology, the animal antibody gene can be deleted. The human antibody gene is then transferred to the antibody gene-deficient animal through transgenic or trans-chromosomal technology, which will express the human antibodies to achieve complete humanization of the antibody.
Using animal gene knockout and insertion to obtain antibodies is difficult and costly, and there is still a human rejection, so phage display technology emerged as times required. The variable region gene of the human antibody is inserted into the appropriate position of the phage coat protein structural gene, which is expressed along with the expression of the phage coat protein, and meanwhile, it is displayed on the phage surface with the reassembly of the phage. Fully human antibodies are then obtained through display library screening and cell expression.
Fully human antibodies have minimal immunogenicity to the human body and a wide range of applications in the treatment of diseases such as cancer, and are of great research and production value.
As the name implies, small-molecule antibodies are antibodies with smaller molecular weights, generally a part of complete Ig. Existing small-molecule antibodies include Fab, Fv, scFv, and single-domain antibody (sdAb).
The molecular weight of small-molecule antibodies is only 1/12~1/2 of the size of a complete Ig, with strong penetrability and antigen affinity, which can be manipulated and edited by genetic engineering systems and mass-produced by various recombinant protein expression systems.
A bispecific antibody has two specific antigen-binding sites and can bind to two different antigens or epitopes simultaneously. For example, they can bind to target cells (cancer cells) and effector cells (T cells) at the same time and mediate the killing effect of effector cells on target cells. The bispecific antibody is a hotspot research object in antibody drugs for their dual-target ability in tumor treatment.
Bispecific antibodies, as antibody drugs with two specific antigen-binding sites, are "antibody bomb" for the tumor treatment with stronger orientation and therapeutic effect than ordinary antibody drugs. Bispecific antibodies can only be obtained by artificial preparation.
Production of Recombinant Antibodies
The production of recombinant antibodies can be divided into five steps:
(1) Building an antibody gene library.
(2) Constructing DNA recombinant phages, eukaryotic cells, or transgenic animals.
(3) Isolating antibodies against target antigens.
(4) Modification of isolated antibodies.
(5) Scaling up production of selected antibodies in expression systems.
Production systems of recombinant antibodies are listed below.
|
|
Growth | Generation of Cells | Yield | Glycosylation | Whole lg |
| In Vitro | |||||
| Reticulocyte Lysate | not necessary | not necessary | Very low | - | - |
| Prokaryotic Organisms | |||||
| E.coli | |||||
| - Cytoplasm | Very fast | Simple | High/Req. S--S refolding | - | - |
| - Periplasm, Soluble | Very fast | Simple | Low/Medium | - | - |
| - Periplasm, Inclusion Bodies | Very fast | Simple | High/Req. refolding | - | - |
| Other Gram-Negative | Very fast | Simple | Low/Medium | ||
| Gram-Positive | Fast | Simple | Low/Medium | - | - |
| Eukaryotic Organisms | |||||
| Yeasts | Medium | 1-n months | Medium | Different/Engineered | |
| Filamentous Fungi | Medium | > 1 month | Low/Medium | Different | + |
| Baculovirus/Insect Cells | Medium | > 1 month | Low | Different | + |
| Mammalian Cells | |||||
| - Transient | Medium | Simple | Medium | + | + |
| - Stable | Medium | Ca. 1 year | High | + | |
| Transgenic Plants | Slow | 6 months – > 1 year | High | Different | + |
| Transgenic Animals | Slow | > 1 year | High | + | + |
Recombinant Antibody Benefits
Compared with conventional monoclonal antibodies produced by hybridoma technology, recombinant antibodies reserve multiple benefits:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Easy mass production
• Sustainable supply
• Animal-free production
The table below shows a comparison between polyclonal, monoclonal, and recombinant antibodies.
| Polyclonal Antibody | Monoclonal Antibody | Recombinant Antibody | |
| Production | |||
| - Time | Short (3–4 months) | Long (up to a year) | Short to moderately long |
| - Cost | Low | Moderate to high | Low to high |
| - Ease | Very easy | Difficult | Easy |
| Targets | Immunogenic targets | Immunogenic targets | No limitations |
| Stability | High | Moderate | Depends on the format |
| Reproducibility | Limited | Virtually reproducible | Fully reproducible |
| Specificity | Moderate | High | High |
| Sensitivity | Variable | Moderate to high | High |
| Engineering | Not possible | Only After converting to rAb | Possible |
| Availability | Commercially available | Commercially available | Limited commercial availability |
Advantages of Creative Biolabs' Hi-Affi™ Recombinant Antibody
We have developed proprietary procedures for Hi-Affi™ recombinant antibody production, with optimized library construction and screening procedures using hybridoma or phage display technology. We guarantee successful isolation of clones in a short time with high affinity up to the sub-nanomolar level. Bulk order from 1 mg to 10 g is also accepted. Advantages of our Hi-Affi™ recombinant antibodies are:
• High purity (>98%)
• High affinity
• Broad applications
• Antibody engineering possible
• Reproducibility
• Complete condition control
• Short production time
• No animal required
Customer Testimonials
Creative Biolabs is a pioneer of antibody discovery and manufacture, providing the most comprehensive list of recombinant antibody products. See customers' testimonials on our products in the video below, which demonstrates the benefits of recombinant antibodies and why you should pick our Hi-Affi™ recombinant antibody products to boost your antibody therapy research.
References
- Schirrmann, T., Al-Halabi, L., Dübel, S., & Hust, M. (2008). Production systems for recombinant antibodies. Frontiers in bioscience : a journal and virtual library, 13, 4576–4594.
- Anchal Singh, Ayushi Mishra, Anju Verma. (2020). Chapter 17. Antibodies: monoclonal and polyclonal. Animal Biotechnology, 339-360.
- Peltomaa, R., Barderas, R., Benito-Peña, E., & Moreno-Bondi, M. C. (2022). Recombinant antibodies and their use for food immunoanalysis. Analytical and bioanalytical chemistry, 414(1), 193–217.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.