

Briakinumab Overview
Introduction of Briakinumab
Briakinumab, also known as ABT-874, is a therapeutic antibody targeting at interleukin-12 (IL-12) and interleukin-23 (IL-23). It was developed by the Abbott Laboratories for the treatment of a number of T-cell driven autoimmune diseases such as crohn's disease, psoriasis, rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. However, the development of briakinumab in the treatment of psoriasis has been discontinued in the U.S. and Europe as of 2011. The reason is that researchers had found there were adverse cardiovascular events associated with the briakinumab’s clinical treatment trials of severe psoriasis. As the same time, non-melanoma skin cancers also happens in the treatment group. Due to these severe adverse events in the treatment, Abbott withdrew its application for briakinumab from the USA FDA and European Medicines Agency and was evaluating next steps. So far, 10 trials in the clinical laboratory database have been completed, except one is withdrawn. Most of these studies are about the treatment of psoriasis, although the results show that the efficacy is good, its safety has become an important issue hindering its further research and development.
Mechanism of Action of Briakinumab
Briakinumab is a fully human monoclonal antibody directed against the p40 subunit shared by IL-12 and IL-23, two inflammation factors associated with autoimmune disease. Both IL-12 and IL-23 are cytokines belonged to the IL-12 family, which plays important roles in shaping immune responses during antigen presentation and influence cell-fate decisions of differentiating naïve T cells, and regulating functions of a variety of effector cells. Thus, IL-12 family cytokines is always considered as the potential therapeutic targets. IL-12 is naturally produced by dendritic cells (DCs), macrophages, neutrophils, and human B-lymphoblastoid cells (NC-37) and is the first described member of IL-12 family. IL-12 is a heterodimeric cytokine comprised of IL-12p35 and IL-12p40 subunits. The binding of IL-12 to IL-12 receptor (IL-12Rβ1/IL-12Rβ2) activates TYK2 (tyrosine kinase 2), JAK2 and STAT pathways. Il-12 is found to involve in the differentiation of naive T cells into Th1 cells and the activities of natural killer cells and T lymphocytes. In addition, IL-12 also has anti-angiogenic activity. IL-23 is also a heterodimeric cytokine composed of an IL12B (IL-12p40) subunit and the IL23A (IL-23p19) subunit, and its high-affinity receptor is IL-23 receptor (IL-12Rβ1/IL-23R). Upon binding, the complex of IL-12 and IL-23 recruits of janus kinase 2 and tyrosine kinase 2 kinases, which transduce the signal and activate the STAT3 and STAT4 signal pathways. It was found that IL-23 prolongs the expression of type 17 signature cytokines (such as IL-17, IL-22 and GM-CSF) that induce tissue pathology and mediates chronic inflammation by promoting the survival and maintenance of Type 17 cells. Il-23 was found in a variety of pathological tissues such as the skin of patients with psoriasis, in the bowel wall of patients with chronic inflammatory bowel disease, and in synovial membrane of patients with rheumatoid arthritis. Briakinumab is designed as a monoclonal antibody targets and neutralizes IL-12 and IL-23. As an inhibitor, it can bind to the p40 subunit of IL-12 and IL-23, blocking its binding at the receptor and the subsequent downstream signaling.
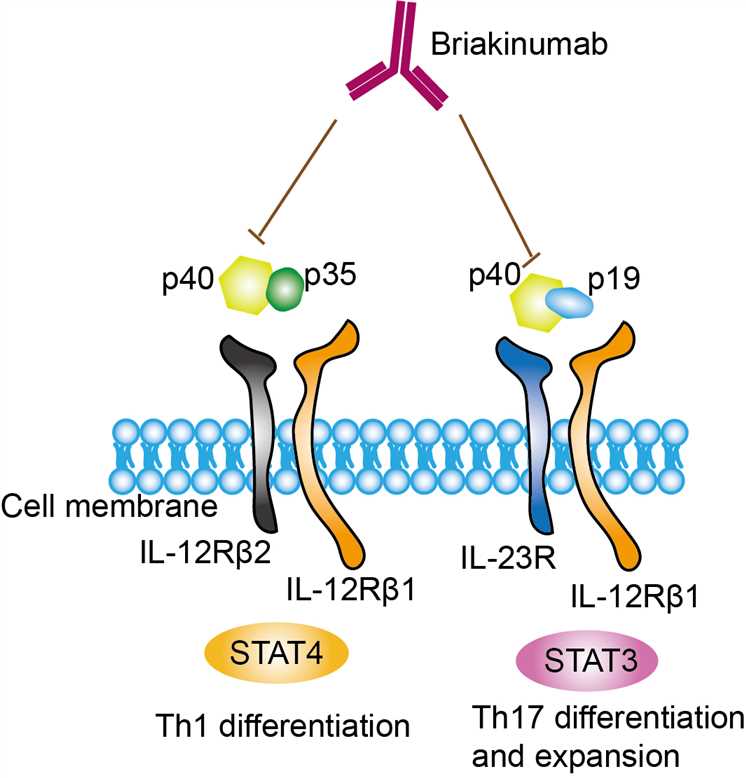 Fig.1 Mechanism of Action of Briakinumab
Fig.1 Mechanism of Action of Briakinumab
What We Provide
Therapeutic Antibody
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

