Afuco™ Anti-Human SHH ADCC Recombinant Antibody (MEDI-5304), ADCC Enhanced (CAT#: AFC-544CL)
Anti-SHH ADCC Enhanced Antibody (MEDI-5304) is an ADCC enhanced antibody produced by our Afuco™ platform. MEDI-5304 is the first fully human hedgehog antibody which inhibited transcription of hedgehog target genes and osteoblast differentiation of C3H10T1/2 cells. MEDI-5304 represents a potent dual hedgehog inhibitor suitable for continued development to evaluate efficacy and safety in human patients with tumors harboring elevated levels of SHH or IHH.

Figure 1 Pharmacokinetics and pharmacodynamics of MEDI-5304.
A, MEDI-5304 displays biologic activity in vivo. Ncr nude mice bearing Colo205 xenograft tumors were treated intraperitoneally with vehicle or varying concentrations of antibody for 24 hours. RNA expression of mGLI1 and mPTC1 in excised tumors was determined by qRT-PCR. Values represent mean ± SEM. Statistical analysis was performed using one-way ANOVA and Dunnett multiple comparison tests, and significance (***, P < 0.001) is indicated when comparing antibody treatment to vehicle controls. B, PK/PD relationship of MEDI-5304 in athymic mice bearing Colo205 xenograft tumors. Athymic mice with approximately 200 mm3 Colo205 tumors were treated with vehicle (t = 0) or a single 1 mg/kg i.p. dose of MEDI-5304. Tumors were excised at various times and relative mGLI1 and mPTC1 RNA levels were determined. Serum levels of MEDI-5304 were determined with a human IgG-specific ELISA.
Michaud, N. R., Wang, Y., McEachern, K. A., Jordan, J. J., Mazzola, A. M., Hernandez, A., ... & Wang, L. (2014). Novel neutralizing hedgehog antibody MEDI-5304 exhibits antitumor activity by inhibiting paracrine hedgehog signaling. Molecular cancer therapeutics, 13(2), 386-398.

Figure 2 MEDI-5304 exhibits single agent and combination efficacy with carboplatin in a coimplant model of paracrine hedgehog signaling.
A, MEDI-5304 inhibits the growth of HT-29/MEF coimplant tumors. HT-29 and MEFs were mixed (1:5 cell ratio) and implanted subcutaneously into Ncr nude mice, which were dosed (10 animals/group) intraperitoneally with varying amounts of MEDI-5304, 10 mg/kg 5E1 or 10 mg/kg R347 twice weekly starting on day 11. Tumor volumes are geometric mean ± SEM. Student t test was used to determine significance of MEDI-5304 or 5E1 treatment relative to R347 treatment on day 35 (*, P < 0.05). B and C, end of efficacy study pharmacodynamic effects of MEDI-5304 and 5E1 on hedgehog target gene expression in tumor stroma (B) and mouse skin (C). Tumors were harvested and skin samples were obtained 24 hours after last dose and RNA expression of stromal hedgehog target and pathway genes (mGLI1, mGLI2, mGLI3, mPTC1, mPTC2, mHHIP, mIGFBP-5, and mSMO) was measured using qRT-PCR. Values represent mean ± SEM. One-way ANOVA and Dunnett multiple comparison tests were used to determine statistical significance (*, P < 0.05) of antibody treatment compared with the R347 control group. D, MEDI-5304 enhances tumor growth inhibition of carboplatin treatment of the HT-29/MEF coimplant model. Mice with HT-29/MEF coimplant tumors received MEDI-5304 (10 mg/kg twice/week i.p.), carboplatin (30 mg/kg once/week i.p.), or both agents in combination for 4 weeks starting on day 12. Tumor volumes are geometric mean ± SEM. Student t test was used to determine statistical significance of combination treatment compared with R347 (***, P < 0.001) and significance of combination treatment compared with each agent alone (*, P < 0.05).
Michaud, N. R., Wang, Y., McEachern, K. A., Jordan, J. J., Mazzola, A. M., Hernandez, A., ... & Wang, L. (2014). Novel neutralizing hedgehog antibody MEDI-5304 exhibits antitumor activity by inhibiting paracrine hedgehog signaling. Molecular cancer therapeutics, 13(2), 386-398.

Figure 3 MEDI-5304 lacks effects on tumor growth and CSCs in primary pancreatic explants models.
A, MEDI-5304 lacks antitumor activity against three primary pancreatic explant models. RAG2 knockout mice (10 mice/group) with subcutaneous P479 tumors were treated with R347 (10 mg/kg), MEDI-5304 (10 or 30 mg/kg), or gemcitabine (60 or 120 mg/kg) given intraperitoneally twice a week starting on day 16. Animals with orthotopic tumors from models 890 and 947 (8 mice/group) were untreated or received MEDI-5304 (10 mg/kg), gemcitabine (120 mg/kg), or both treatments in combination. Student t test was used to determine significance of each treatment compared with the untreated control (*, P < 0.05; ***, P < 0.001). B, MEDI-5304 has no effect on CSC frequency in primary pancreatic tumor models. End of study samples were collected 24 hours after last dose on a minimum of three samples per treatment arm and flow cytometry analysis of the CSC population (ESA+, CD44+, and CD24+) was performed. Data are presented as % CSC, the percentage of cells staining positive for all three markers. Statistical analysis was performed using one-way ANOVA and Dunnett multiple comparison tests (**, P < 0.01; ***, P < 0.001). C, MEDI-5304 does not improve responsiveness to gemcitabine pretreatment or coadministration. Mice with subcutaneous P479 tumors were either untreated (closed black squares) or treated intraperitoneally with 120 mg/kg gemcitabine every 4 days for four doses (closed green squares). Animals receiving gemcitabine were then re-randomized on day 29 into groups of 10 mice/group, which then received one of the following treatment regimen every 4 days: 10 mg/kg R347 (closed black triangles), 120 mg/kg gemcitabine (closed green squares), 1 mg/kg MEDI-5304 (closed blue circles), 10 mg/kg MEDI-5304 (open blue circles), combination of 120 mg/kg gemcitabine and 1 mg/kg MEDI-5304 (closed red diamonds), or combination of 120 mg/kg gemcitabine and 10 mg/kg MEDI-5304 (closed purple diamonds). Tumors in mice that did not receive gemcitabine during the second dosing phase grew to approximately 1,000 mm3 and these mice were sacrificed on day 50. The second phase of dosing for animals that received gemcitabine as part of the dosing regimen was discontinued on day 53, and outgrowth of those tumors was monitored until day 71. No differences among these three groups were detected. D, MEDI-5304 does not affect P479 tumorpshere growth. Dissociated cells from P479 tumors were plated in media that promotes tumorsphere formation. Cells were plated in the absence or presence of 10 or 30 μg/mL negative control antibody R347, 10 or 30 μg/mL MEDI-5304, 5 or 10 μmol/L cyclopamine, or salinomycin (2 μmol/L). Statistical analysis was performed using one-way ANOVA and Dunnett multiple comparison tests. Statistical significance (***, P < 0.001) is indicated for salinomycin in comparison with the DMSO control.
Michaud, N. R., Wang, Y., McEachern, K. A., Jordan, J. J., Mazzola, A. M., Hernandez, A., ... & Wang, L. (2014). Novel neutralizing hedgehog antibody MEDI-5304 exhibits antitumor activity by inhibiting paracrine hedgehog signaling. Molecular cancer therapeutics, 13(2), 386-398.

Figure 4 Effects of HH ligand depletion or pharmacologic HH inhibition on RMS cells invasiveness.
Cell invasiveness was studied after genetic (shRNAs indicated) and pharmacologic (Vismodegib 50 μM and MEDI-5304 30 μg/ml, 3 days of treatment) HH pathway inhibition. The cell lines were RD (A), RH4 (B) and RH30 (C). Values of invasiveness are expressed in percentages and referred to those of controls (empty vector or vehicle). (D) Overall survival of mice after Vismodegib treatment was reduced compared to mice treated with vehicle. All mice were intravenously injected with 10⁶ RH30 cells. Significance: *P<0.05, **P<0.01, ***P<0.001.
Almazán-Moga, A., Zarzosa, P., Molist, C., Velasco, P., Pyczek, J., Simon-Keller, K., ... & Soriano, A. (2017). Ligand-dependent Hedgehog pathway activation in rhabdomyosarcoma: The oncogenic role of the ligands. British journal of cancer, 117(9), 1314.

Figure 5 Effects of the depletion of HH ligands on cell proliferation.
(A, C and E) Relative proliferation, expressed as a reduction in WST uptake, compared to control cells (100%). The experiments were performed in the following samples: control (empty vector or vehicle) cells, cells transduced with shRNAs for SHH, IHH, DHH and GLI1 and, finally, cells treated with Vismodegib (50 μM) or the HH blocking antibody MEDI-5304 (30 μg/ml) after 4 days. The RMS cell lines analysed were RD, RH4 and RH30.
Almazán-Moga, A., Zarzosa, P., Molist, C., Velasco, P., Pyczek, J., Simon-Keller, K., ... & Soriano, A. (2017). Ligand-dependent Hedgehog pathway activation in rhabdomyosarcoma: The oncogenic role of the ligands. British journal of cancer, 117(9), 1314.
Specifications
- Host Species
- Human
- Derivation
- Human
- Type
- ADCC enhanced antibody
- Species Reactivity
- Human
- Related Disease
- Tumors
Product Property
- Purity
- >95% as determined by Analysis by RP-HPLC & analysis by SDS-PAGE
- Storage
- 4 °C, -20°C if preferred
Target
- Alternative Names
- SHH; sonic hedgehog; TPT; HHG1; HLP3; HPE3; SMMCI; TPTPS; MCOPCB5; sonic hedgehog protein; sonic hedgehog homolog
- Gene ID
- 6469
- UniProt ID
- Q15465
Related Resources
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Downloads
Download resources about recombinant antibody development and antibody engineering to boost your research.
See other products for "SHH"
scFv Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-902-S(P) | Recombinant Anti-human SHH Antibody scFv Fragment | IP, IF, Biosensors, FuncS | scFv |
| MHH-902-S(P) | Recombinant Human Anti-human SHH Antibody scFv Fragment | ELISA, WB, IF, FuncS | scFv |
| PSBL-696 | Mouse Anti-SHH Recombinant Antibody (clone 5E1); scFv Fragment | Neut | Mouse scFv |
| HPAB-0710-CN-S(P) | Human Anti-SHH Recombinant Antibody (clone 3H8); scFv Fragment | ELISA, FC | Human scFv |
| HPAB-N0236-YC-S(P) | Mouse Anti-SHH Recombinant Antibody; scFv Fragment (HPAB-N0236-YC-S(P)) | ELISA, FC, WB, FuncS | Mouse scFv |
Recombinant Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MHH-902 | Recombinant Human Anti-human SHH Antibody | WB, IHC, FuncS | IgG |
| MOB-1591MZ | Recombinant Mouse Anti-Human SHH Antibody (clone 20I7) | ICC/IF, WB, sELISA | Mouse antibody |
| HPAB-N0236-YC | Mouse Anti-SHH Recombinant Antibody (HPAB-N0236-YC) | ELISA, FC, WB, FuncS, Inhib, IF | Mouse IgG |
| HPAB-AP788-YC | Human Anti-SHH Recombinant Antibody (clone 1A12) | ELISA, Inhib | Human IgG |
| HPAB-AP789-YC | Human Anti-SHH Recombinant Antibody (clone 1G12) | ELISA, Inhib | Human IgG |
Human Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-566CL | Anti-Human SHH Recombinant Antibody (MEDI-5304) | Inhib | Antibody |
Fab Fragment Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-696 | Human Anti-SHH Recombinant Antibody (clone 5E1); Fab Fragment | Neut | Fab |
| HPAB-AP788-YC-F(E) | Human Anti-SHH Recombinant Antibody (clone 1A12); Fab Fragment | ELISA, Inhib | Human Fab |
| HPAB-AP789-YC-F(E) | Human Anti-SHH Recombinant Antibody (clone 1G12); Fab Fragment | ELISA, Inhib | Human Fab |
| HPAB-AP790-YC-F(E) | Human Anti-SHH Recombinant Antibody (clone 1H10); Fab Fragment | ELISA, Inhib | Human Fab |
| HPAB-AP791-YC-F(E) | Human Anti-SHH Recombinant Antibody (clone 4B6); Fab Fragment | ELISA, Inhib | Human Fab |
Neutralizing Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1955CQ | Recombinant Rat Anti-Shh Antibody (6K12) | IHC, Neut, WB | IgG2 |
| NEUT-1956CQ | Recombinant Rat Anti-Shh Antibody (CBL167) | IHC, Neut, WB | IgG2 |
| NEUT-1957CQ | Recombinant Rat Anti-Shh Antibody (CBL886) | Neut, WB | IgG2a |
| NEUT-1958CQ | Recombinant Mouse Anti-SHH Antibody (171018) | IHC-FoFr, WB, IHC-Fr, Neut, ELISA, ICC/IF | IgG2a |
Rabbit Monoclonal Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-3261 | Hi-Affi™ Recombinant Rabbit Anti-SHH Monoclonal Antibody (DS3261AB) | FC, IHC-P, IP, WB | IgG |
| MOR-4739 | Hi-Affi™ Recombinant Rabbit Anti-SHH Monoclonal Antibody (TH253DS) | WB, IF, ICC, FC | IgG |
Neuroscience Antibody
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NS-082CN | Human Anti-SHH Recombinant Antibody (clone 6D7) | ELISA, FC | Human IgG2 |
| NS-082CN-F(E) | Human Anti-SHH Recombinant Antibody (clone 6D7); Fab Fragment | ELISA, FC | Human Fab |
| NS-082CN-S(P) | Human Anti-SHH Recombinant Antibody (clone 6D7); scFv Fragment | ELISA, FC | Human scFv |
Customer Reviews and Q&As
There are currently no Customer reviews or questions for AFC-544CL. Click the button above to contact us or submit your feedback about this product.
View the frequently asked questions answered by Creative Biolabs Support.
For Research Use Only. Not For Clinical Use.
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


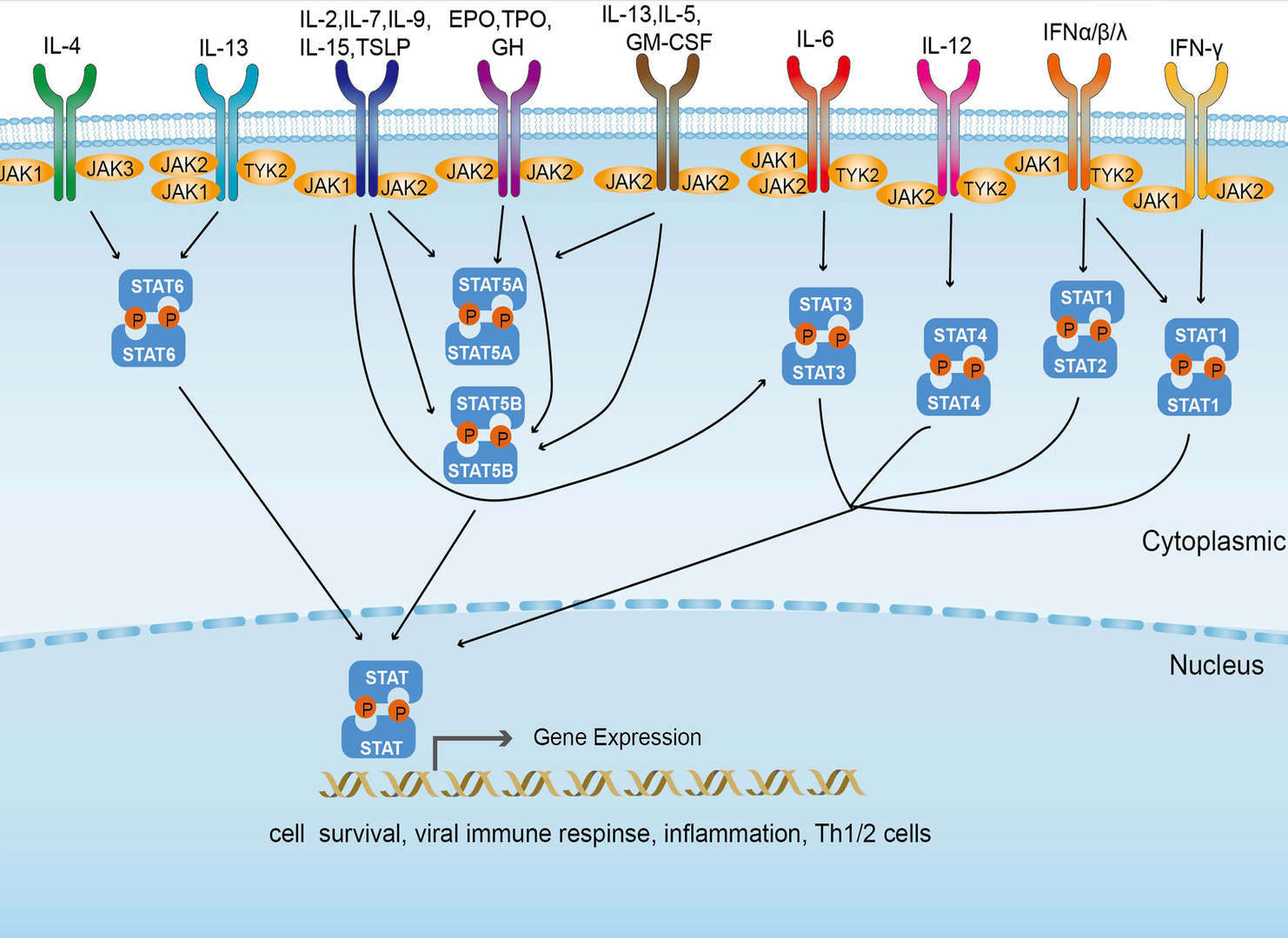 JAK-STAT Signaling Pathway
JAK-STAT Signaling Pathway
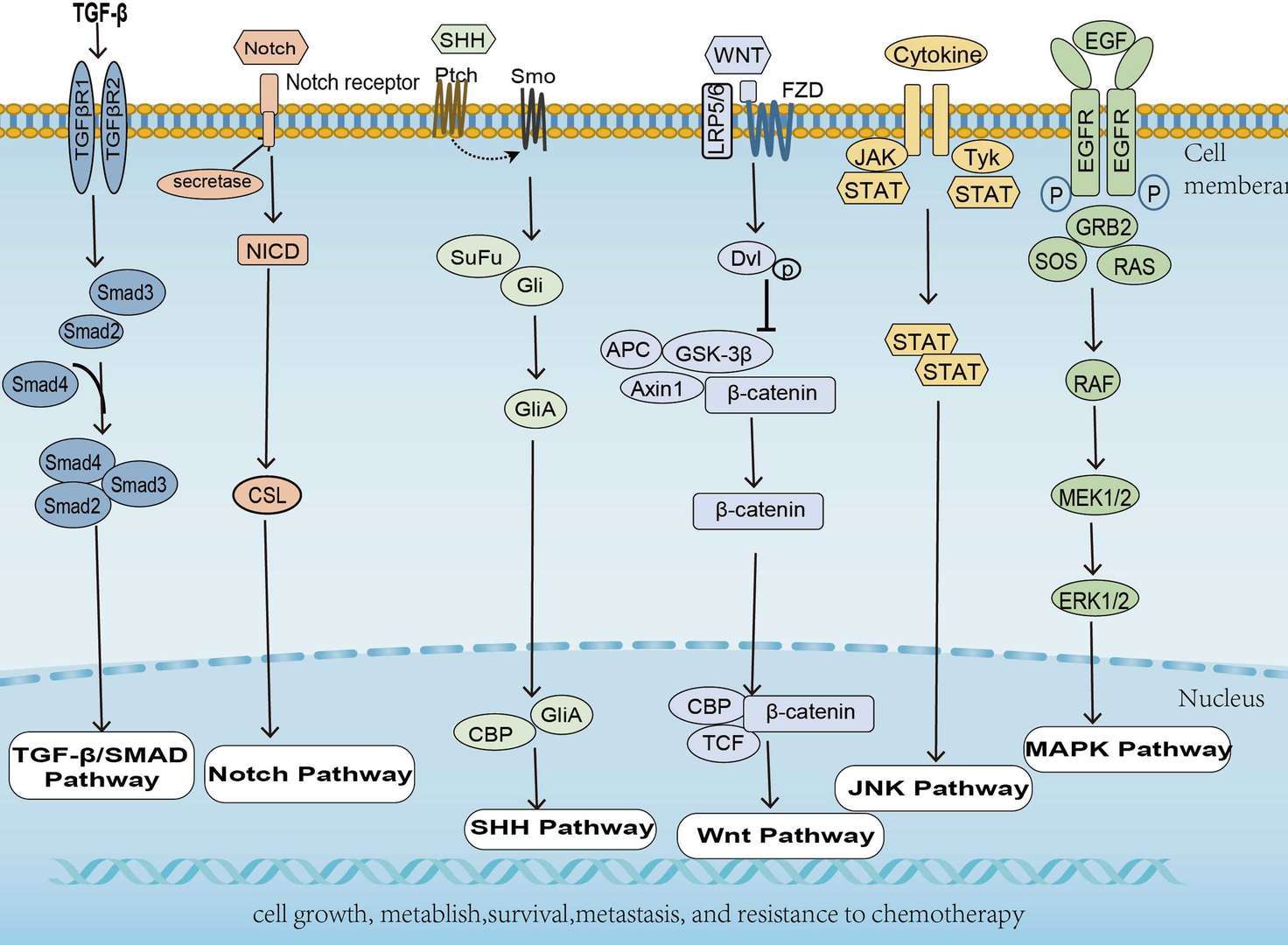 Pancreatic Cancer
Pancreatic Cancer
