

Bleselumab Overview
Introduction of Bleselumab
Bleselumab (also known as ASKP1240) is a fully human monoclonal antibody (mAb) which is designed for the prevention of organ transplant rejection and as an alternative to anti-CD154 mAb. This drug was developed by Kyowa Kirin Pharmaceutical Development Inc. It is an IgG4 masking antibody that shows neither antibody-dependent cell-mediated cytotoxicity (ADCC) nor complement-dependent cytotoxicity (CDC). This ASKP1240 antibody was recently reported to significantly prolong kidney, liver and islet graft survival in nonhuman primates. The current study characterized this antibody with respect to its effects on soluble human CD154 (shCD154) induced cellular proliferation. In vitro, bleselumab inhibited shCD154-stimulated cellular proliferation demonstrating its ability to block CD40–CD154 interactions on a cellular level and it is not prothromboembolic in that it did not inhibit collagen-induced stable thrombus formation, nor did it destabilize the thrombus once formed under flow conditions. In vivo, bleselumab administration suppressed indicators of both cell-mediated and antibody immune responses (DTH and anti-TTx antibody responses). The safety studies showed that the weekly administration of ASKP1240 at doses up to 100 mg/kg was not immunogenic, and resulted in no significant toxicity. Based on these findings, it is concluded that the fully human antagonistic CD40 mAb, bleselumab, is a good candidate for further clinical development in transplant and autoimmune diseases.
Mechanism of Action of Bleselumab
A myriad of studies over the past two decades have revealed the importance of CD40-CD154 interactions in the generation of alloreactive responses, and as such it represents an extremely attractive target for therapeutic intervention in transplantation. This pathway requires the interaction of the CD40 molecule with its ligand, CD40L (CD154). These molecules belong to the tumor necrosis factor (TNF) superfamily and are expressed on a wide range of tissues and cell types, with CD40 constitutively expressed on antigen-presenting cells (APC), including B cells, macrophages and dendritic cells (DC), and CD154 inducibly expressed mainly on CD4+ T cells and endothelial cells following activation. The first descriptions of this pathway focused on the importance of CD40-CD154 interactions in promoting T cell-dependent humoral responses, but it quickly became apparent that these interactions were also essential for generating cell-mediated immunity. Seminal studies showed that the crosslinking of CD40 on dendritic cells by CD154 on activated CD4+ helper T cells led to “licensing” of the APC, leading to the upregulation of the B7 family of costimulatory molecules and the elaboration of proinflammatory cytokines. More recent studies have also suggested that the interaction of CD154 on activated CD4+ T cells and CD40 on CD8+ T cells also plays a critical role in generating effective cytotoxic T cell responses. The anti-CD40 therapeutic that is furthest along in the clinical translation pipeline is bleselumab. This drug inhibits immune responses by blocking CD40-CD154 interaction between T cells, B cells, and antigen-presenting cells. Initially described in both renal and islet transplantation models in cynomolgus monkeys, bleselumab showed minimal efficacy when given as induction therapy, and also caused significant depletion of peripheral B cells. However, maintenance treatment led to long-term kidney allograft survival, as well as islet allograft survival as a monotherapy, and also prevented donor-specific antibody formation. These exciting results led to the initiation of clinical trials, and phase I studies in humans indicated that this therapy was well-tolerated and effective at achieving CD40 receptor occupancy at doses similar to those used in non-human primates, suggesting that this is a promising avenue for use in human autoimmune disease and transplantation.
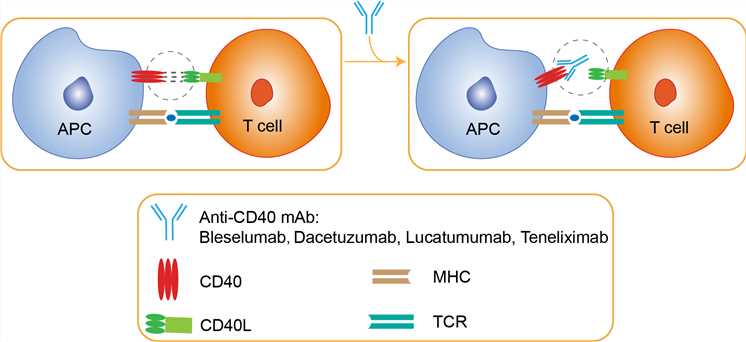
Fig 1. Mechanism of Action of Bleselumab
Table 1. Clinical Projects of Bleselumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02921789 | Recruiting | Kidney Transplantation, Focal Segmental Glomerulosclerosis (FSGS) | Astellas Pharma Global Development, Inc. | October 3, 2016 |
What We Provide
Therapeutic Antibody
Bleselumab
We provide high-quality Bleselumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?term=Bleselumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

