

Cabiralizumab Overview
Introduction of Cabiralizumab
Cabiralizumab (FPA008) is a humanized monoclonal antibody directed against colony stimulating factor-1 receptor (CSF1R), which is a member of the CSF1/PDGF receptor family of tyrosine-protein kinases. The developers of cabiralizumab including Apexigen, Bristol-Myers Squibb, Five Prime Therapeutics, University of Chicago and Yale University. Although it is reported to be designed for the treatment of tenosynovial giant cell tumor, the studies of treatment for pancreatic cancer, pigmented villonodular synovitis, malignant melanoma, non-small cell lung cancer, renal cell carcinoma and solid tumors also have been reported to be undergoing. The trails of pancreatic cancer and pigmented villonodular synovitis are in Phase II, while others are in Phase I. Orphan designation of cabiralizumab had been granted in the United States and European Commission for the treatment of pigmented villonodular synovitis.
Mechanism of Action of Cabiralizumab
CSF1R, also known as macrophage colony-stimulating factor receptor (M-CSFR) and CD115 (Cluster of Differentiation 115), is a receptor for hematopoietic growth factor called CSF1. Binding of CSF1R to its ligand CSF1R, regulates the proliferation, differentiation, and survival of various cells including monocytes, macrophages, bone marrow progenitor cells and earlier hematopoietic progenitors. The mutation of CSF1R is related to chronic myelomonocytic leukemia and type M4 acute myeloblastic leukemia. And the high expression levels of cabiralizumab are found in many cancers and on tumor-associated macrophages (TAMs). Thus, it has been considered as an important therapeutic target for cancer or inflammatory diseases. Cabiralizumab is developed as a monoclonal antibody antagonist targeted CSF1R, with potential antineoplastic activity. Upon administration, the drug inhibits the binding of CSF1R to CSF1 and interleukin-34 (IL-34) via binding to CSF1R expressed on monocytes, macrophages, and osteoclasts competitively. This inhibits the activation of CSF1R and blocks the signaling transduction mediated by CSF1R in these cells, decreasing the production of inflammatory mediators by macrophages and monocytes and reduces inflammation. Cabiralizumab exerts its anti-tumor function by repressing the activity of CSF1R-dependent tumor-associated macrophages (TAMs), which play important roles in tumor cell proliferation, tumor angiogenesis, invasion and metastasis, immunosuppression, and drug resistance. In addition, cabiralizumab blocks the recruitment of TAMs to the tumor microenvironment, leading to the enhancement of T-cell infiltration and antitumor T-cell immune responses and the inhibition of the proliferation of tumor cells. Additionally, cabiralizumab protects bone from destruction by prevents the activation of osteoclasts.
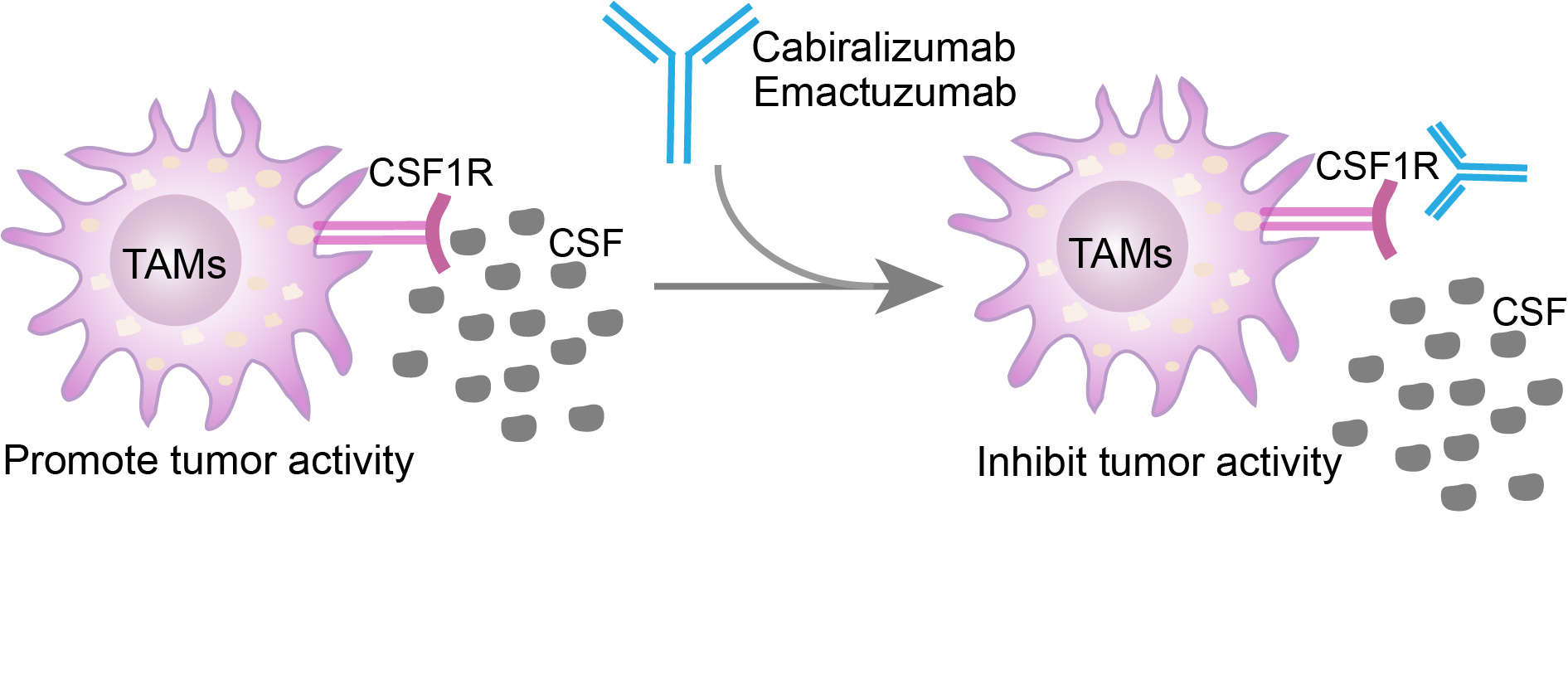 Fig. 1 Mechanism of action of Cabiralizumab
Fig. 1 Mechanism of action of Cabiralizumab
Table 1. Clinical Projects of Cabiralizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03599362 | Recruiting | Pancreatic Cancer | New York University School of Medicine | September 3, 2018 |
| NCT03697564 | Not yet recruiting | Pancreatic Cancer Stage IV | Hitendra Patel | October 5, 2018 |
| NCT02471716 | Active, not recruiting | Pigmented Villonodular Synovitis; Tenosynovial Giant Cell Tumor | Five Prime Therapeutics, Inc. | October 1, 2018 |
| NCT03502330 | Recruiting | Advanced Melanoma; Non-small Cell Lung Cancer; Renal Cell Carcinoma | Yale University | June 19, 2018 |
| NCT02526017 | Active, not recruiting | Advanced Solid Tumors; Including But Not Limited to Lung Cancer; Head and Neck Cancer; Pancreatic Cancer; Ovarian Cancer; Renal Cell Carcinoma; Malignant Glioma | Five Prime Therapeutics, Inc. | October 1, 2018 |
| NCT03158272 | Recruiting | Advanced Malignancies | Bristol-Myers Squibb | October 3, 2018 |
| NCT03336216 | Recruiting | Advanced Pancreatic Cancer | Bristol-Myers Squibb | October 3, 2018 |
| NCT03431948 | Recruiting | Cancer | University of Chicago | June 7, 2018 |
| NCT03335540 | Recruiting | Advanced Cancer | Bristol-Myers Squibb | October 4, 2018 |
What We Provide
Therapeutic Antibody
Cabiralizumab
We provide high-quality Cabiralizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Cabiralizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

