

Camrelizumab Overview
Introduction of Camrelizumab
Camrelizumab (INCSHR-1210, SHR-1210) is an IgG4κ humanized monoclonal antibody (mAb) being investigated for hepatocellular carcinoma. It targets programmed cell death protein 1 (PD-1), a protein on the surface of cells, also known as CD279 (cluster of differentiation 279). Incyte Corporation and Jiangsu Hengrui Medicine Co., Ltd have a global license and collaboration agreement for the development and commercialization of this anti-PD1 antibody. The mAb is being evaluated in the Phase 2/3 (NCT02989922) of patients with advanced hepatocellular carcinoma (HCC) in second-line after failure or intolerance to prior systemic treatment. The study has 2 arms in which patients will be intravenous administered 3 mg/kg SHR-1210 on day 1 every 2 weeks or every 3 weeks. The primary outcome measures are the overall response rate (ORR) and overall survival (OS) rates at 6 months with duration of response and OS at 2 years as secondary endpoints. The estimated enrollment is 220 patients, and the estimated primary completion date is December 2018. A randomized, open-label Phase 3 study (NCT03099382) is evaluating the efficacy of camrelizumab treatment compared to standard-of-care treatment (docetaxel or irinotecan) in patients with esophageal carcinoma. Patients are randomly assigned to receive either SHR-1210 (200 mg every 2 weeks) or the standard of care (docetaxel 75 mg/m2 on day 1 every 3 weeks or irinotecan 180 mg/m2 on day 1 every 2 weeks). This Phase 3 study has an estimated enrollment of 438 and an estimated primary completion date of June 2018.
Mechanism of Action of Camrelizumab
PD-1 is a cell surface receptor that belongs to the immunoglobulin superfamily and is expressed on T cells and pro-B cells. PD-1 binds two ligands, programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2). PD-L1 is also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1), and PD-L2 is also known as cluster of differentiation 273 (CD273). PD-1 is an immune checkpoint and has a role in regulating the immune system's response by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. Physiologically, the PD-1/PD-L1 pathway has emerged as a result of the need to control the degree of inflammation at locations expressing the antigen, in order to secure normal tissue from damage. There is a remarkable expression of the PD-1 protein on the surface of all activated T cells. When a T cell recognizes the antigen expressed by the major histocompatibility complex (MHC) on the target cell, inflammatory cytokines are produced, initiating the inflammatory process. These cytokines result in PD-L1 expression in the tissue, activating the PD-1 protein on the T cell leading to immune tolerance, a phenomenon where the immune system lose the control to mount an inflammatory response, even in the presence of actionable antigens. In certain tumors, most remarkably in melanomas, this protective mechanism is perverted through overexpression of PD-L1; as a result, it circumvents the generation of an immune response to the tumor. PD-1/PD-L1 inhibitors, including camrelizumab, pharmacologically prevent the PD-1/PD-L1 interaction, thus facilitating a positive immune response to kill the tumor.
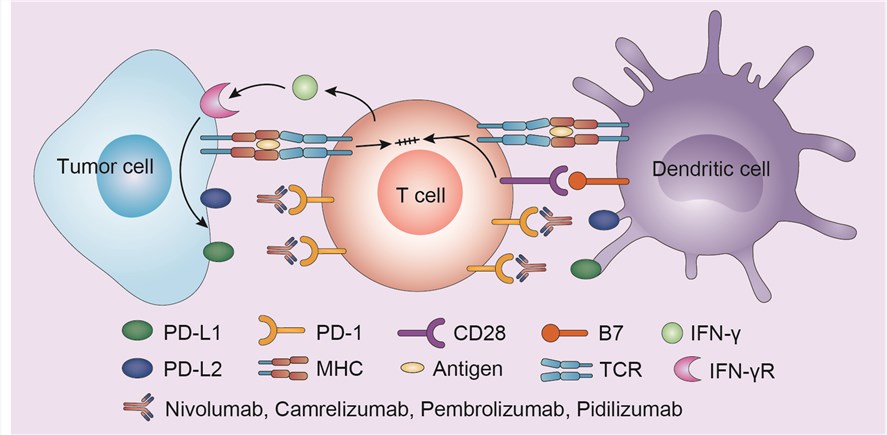 Fig.1 Mechanism of action of camrelizumab
Fig.1 Mechanism of action of camrelizumab
Table 1. Clinical Projects of Camrelizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03707509 | Not yet recruiting | Nasopharyngeal Carcinoma | Jiangsu HengRui Medicine Co., Ltd. | October 16, 2018 |
What We Provide
Therapeutic Antibody
Camrelizumab
We provide high-quality Camrelizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Camrelizumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

