

Farletuzumab Overview
Introduction of Farletuzumab
Farletuzumab (MORAb-003) is a humanized monoclonal antibody (mAb) which is being investigated for the treatment of ovarian cancer. This drug was developed by Morphotek, Inc. It is targeted at Folate receptor alpha (FRα) whose expressed in normal tissues is restricted to a subpopulation of epithelial cells. In contrast, FRα is overexpressed in epithelial ovarian cancer (EOC) and non-small-cell lung carcinoma. Therefore, FRα is considered a promising therapeutic target for EOC and non-small-cell lung carcinoma. Farletuzumab is produced in Chinese hamster ovary (CHO-K1) cells and constructed by grafting complementarity-determining regions (CDRs) of a murine antibody LK26 into a human immunoglobulin G subtype 1 kappa (IgG1/κ) backbone. Farletuzumab is considered to induce immune-dependent cell death, although no underlying mechanism has yet been clarified. Farletuzumab has shown efficacy in both preclinical and clinical studies as a single agent and in combination chemotherapy with minimal drug-specific toxicity. Phase I/II clinical trials clearly demonstrated the feasibility and safety of farletuzumab as a treatment option against solid tumors. However, no improvement in progression-free survival (PFS) was observed in Phase III clinical trials that were conducted to verify the combined effect of cytotoxic drugs and farletuzumab.
Mechanism of Action of Farletuzumab
DNA synthesis in cancer cells is highly dependent on folate, whereas FRα expression in normal tissues is restricted to a subpopulation of epithelial cells. In contrast, FRα is overexpressed in nearly 90% of epithelial non-mucinous ovarian cancers and has been correlated with tumor stage and grade, chemotherapeutic response, and treatment outcome. In addition, FRα expression is retained in recurrent tumors and metastatic lesions. Among lung cancers, FRα expression is increased in non-small-cell lung carcinoma (NSCLC) and is higher in adenocarcinomas than in squamous cell carcinomas. Hence, FRα is considered a promising therapeutic target for epithelial ovarian cancer (EOC) and NSCLC.
In vitro studies found that farletuzumab did not inhibit binding of folic acid and antifolates to FRα and that it had no significant effect on cell growth or folate uptake via FRα. The proposed mechanism of farletuzumab-induced cell toxicity suggests that farletuzumab inhibits FRα-dependent cell growth in a dose-dependent manner and exhibits tumor cytotoxicity through complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (CDCC). Farletuzumab has also been shown to inhibit the interaction between cytoplasmic tyrosine kinase Lyn and membrane-signaling complexes; this results in a reduction in the growth of these cells. A recent preclinical study, which found that farletuzumab inhibited tumor growth via induction of autophagy-associated cell death, demonstrated this inhibitory effect in three-dimensional in vitro models and showed that farletuzumab reduced tumor cell proliferation but had no significant effect on apoptosis, suggesting that blockade of FRα by farletuzumab induced sustained autophagy and suppressed cell proliferation. Anti-tumor activity in animals bearing FRA-expressing ovarian cancers has demonstrated synergy of farletuzumab with taxanes.
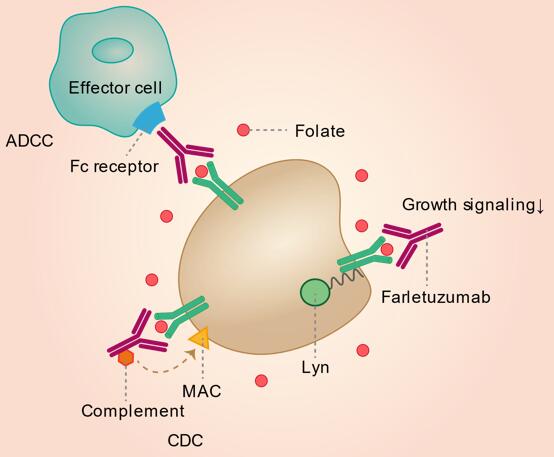 Fig.1 Mechanism of action of farletuzumab
Fig.1 Mechanism of action of farletuzumab
Table 1. Clinical Projects of farletuzumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02289950 | Recruiting | Platinum-Sensitive Ovarian Cancer in First Relapse | Morphotek | November 13, 2014 |
What We Provide
Therapeutic Antibody
Farletuzumab
We provide high-quality Farletuzumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Farletuzumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

