

Hepatocellular Carcinoma Overview - Signaling Pathway. Diagnosis. Targeted Therapy
About Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a primary liver cancer with high mortality, which mainly occurs in patients with liver diseases such as chronic liver disease and cirrhosis. HCC is one of the most common malignant tumors and the second largest cause of death from cancer in the world. Hepatocellular carcinoma is an invasive cancer that spreads to other parts of the liver at a later stage of the disease. There are no specific symptoms in the early stage of hepatocellular carcinoma, but the symptoms in the middle and late stage include liver pain, abdominal distension, fatigue, emaciation, progressive hepatomegaly or epigastric mass. Patients will have hepatomegaly, jaundice, ascites, etc., on signs. In addition, hepatocellular carcinoma will also have some complications, such as upper gastrointestinal bleeding, liver failure and so on. The pathogenic factors of hepatocellular carcinoma are closely related to metabolic syndrome and liver disease. Hepatitis virus infections, such as hepatitis B virus and hepatitis C virus, and non-alcoholic fatty liver disease (NAFLD) are the most common causes of HCC. Cirrhosis develops in 10% of people who drink to excess long time, and 1% to 2% of which develops with HCC. In addition, exposure to carcinogenic substances such as aflatoxin and nitrosamines is also related to the pathogenesis of hepatocellular carcinoma. TP53, PTEN, P16 and mm23, SIRT6 genes are closely related to the occurrence and development of hepatocellular carcinoma. According to the 4th edition of WHO classification of tumors of the digestive system, hepatocellular carcinoma can be divided into 5 morphological subtypes except conventional HCC. They are fibrolamellar HCC (FL-HCC), scirrhous HCC (S-HCC), undifferentiated carcinoma, lymphoepithelioma-like carcinoma and sarcomatoid HCC. The main diagnostic methods of hepatocellular carcinoma are hematological examination and imaging evaluation, such as abdominal ultrasound, computed tomography CT, nuclear magnetic resonance Imaging (NMRI).
Main Signaling Pathways in Hepatocellular Carcinoma
Diagnosis of Hepatocellular Carcinoma
The clinical diagnostic criteria of hepatocellular carcinoma mainly depend on three factors including the background of chronic liver disease, the results of imaging examination, and the level of serum AFP. However, the diagnostic criteria and specific requirements are not unified in academic. For example, liver cirrhosis and hepatitis B virus or hepatitis C virus infection, at the same time CT and MRI imaging show that the liver space occupying diameter≥2cm, can be diagnosed as liver cirrhosis in some criteria. In addition to the above two conditions, other diagnostic criteria also includes serum AFP≥400μg/L for 1 month or 200μg/L for 2 months in patients. Abdominal ultrasonography is the first choice for the diagnosis and screening of hepatocellular carcinoma in high-risk groups. In patients with cirrhosis, the diagnosis of HCC should be based on non-invasive diagnostic criteria or pathological examination, while in non-cirrhotic patients, the diagnosis of HCC should be confirmed by pathology. Serum AFP (alpha-fetoprotein) is the only serum marker widely used in the diagnosis and follow-up of HCC. However, other improved serological markers, whether used alone or in combination with others, are necessary for early HCC diagnosis. Although AFP is the standard for serological markers of HCC, it is not added into the AASLC guidelines because of its poor sensitivity and specificity.
Targeted Therapy for Hepatocellular Carcinoma
Nexavar (Sorafenib) is a polykinase inhibitor that can inhibit tumor cell expansion, angiogenesis and promote tumor cell apoptosis. It can inhibit tumor cell proliferation and angiogenesis by targeting Receptor tyrosine kinases such as serine/threonine kinase (Raf), vascular endothelial growth factor receptor (VEGFR), platelet growth factor receptor β (PDGFR- β), KIT, FLT3 and Ret. Sorafenib in combined with TACE can be used in the treatment of locally late or late HCC, but it is just limited to patients with better liver function as its low objective response rate (ORR) and limited prolonged overall survival (OS). Stivarga (regorafenib) became the first second-line treatment for HCC after the failure of sorafenib, and the approved regorafenib for advanced HCC patients who were resistant to sorafenib or could not tolerate it. The structure and effect of regorafenib are similar to those of sorafenib, but the bioactivity of regorafenib is higher. Lenvima (lenvatinib) is a small molecule polykinase inhibitor, which mainly targets at VEGFR1-3, fibroblast growth factor receptor (FGFR) 1-4, PDGFR α, RET, KIT, and plays important roles in anti-angiogenic. The long-term and short-term effects of lenvatinib are comparable to those of sorafenib. Several countries have approved lenvatinib for the treatment of advanced HCC. Opdivo (nivolumab), an IgG4 antibody targeting human programmed death protein 1 (PD-1), was approved in September 2017 for use in patients with advanced HCC after failed treatment with sorafenib. Its III clinical treatment is under way.
References
1. Malek N P, et al. The diagnosis and treatment of hepatocellular carcinoma. Deutsches Ärzteblatt International, 2014, 111(7): 101.
2. El Jabbour T, et al. Update on hepatocellular carcinoma: Pathologists’ review. World journal of gastroenterology, 2019, 25(14): 1653.
3. Lau W Y. Hepatocellular carcinoma. World Scientific, 2008.
4. Chan S L, et al. Targeted therapy of hepatocellular carcinoma: present and future. Journal of gastroenterology and hepatology, 2012, 27(5): 862-872.
5. Spangenberg H C, et al. Targeted therapy for hepatocellular carcinoma. Nature reviews Gastroenterology & hepatology, 2009, 6(7): 423.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

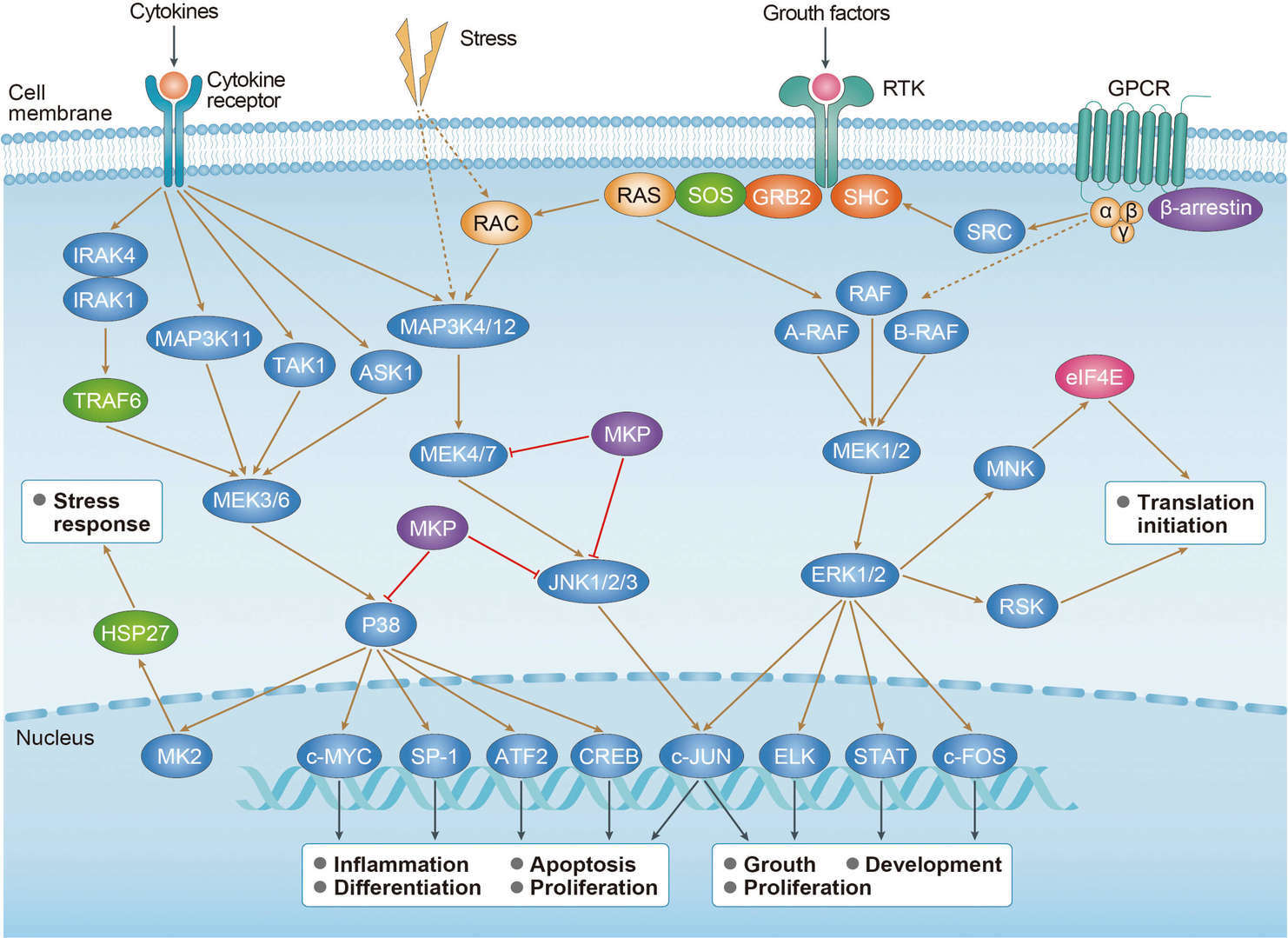 MAPK Pathway
MAPK Pathway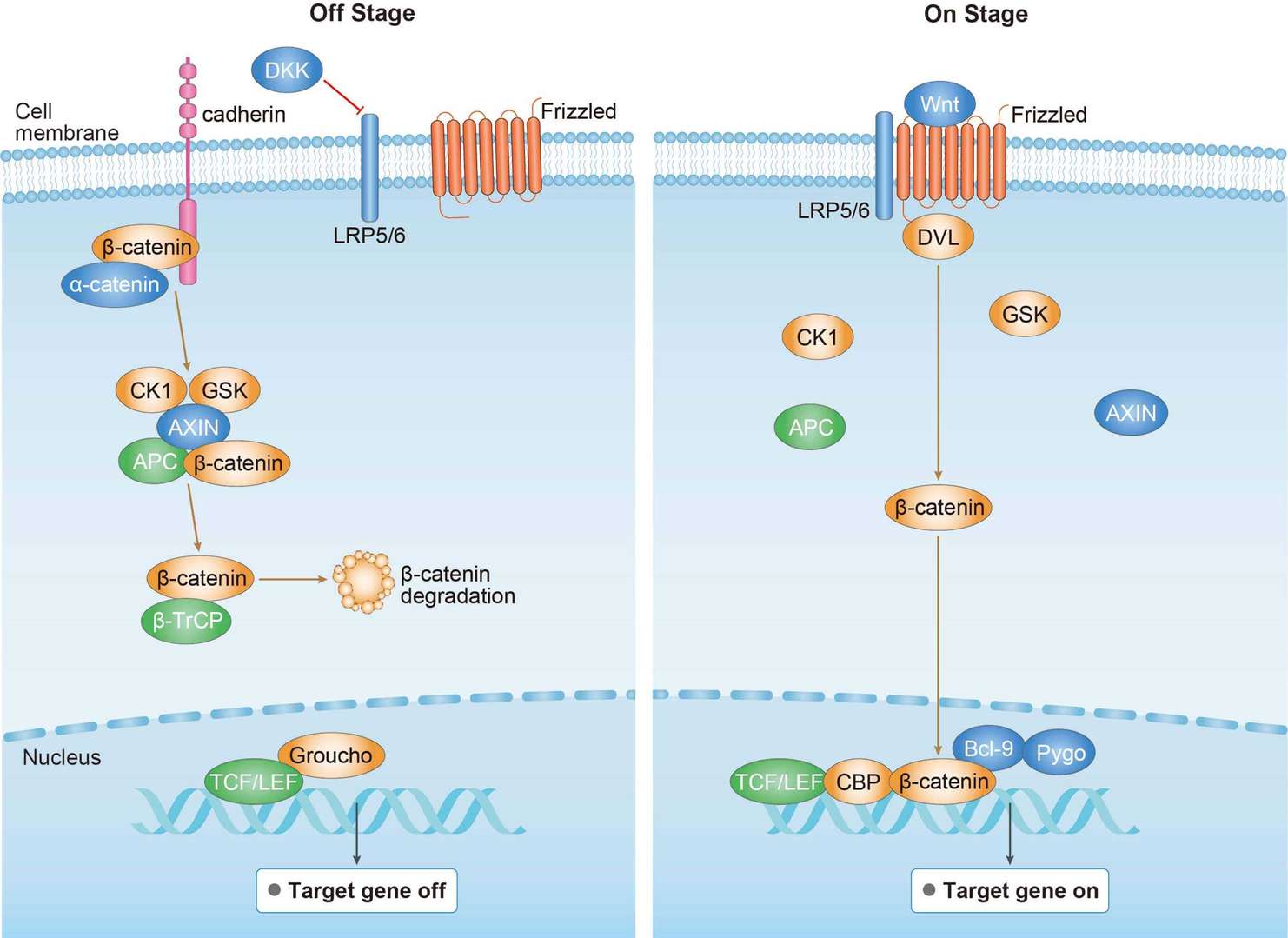 Wnt Pathway
Wnt Pathway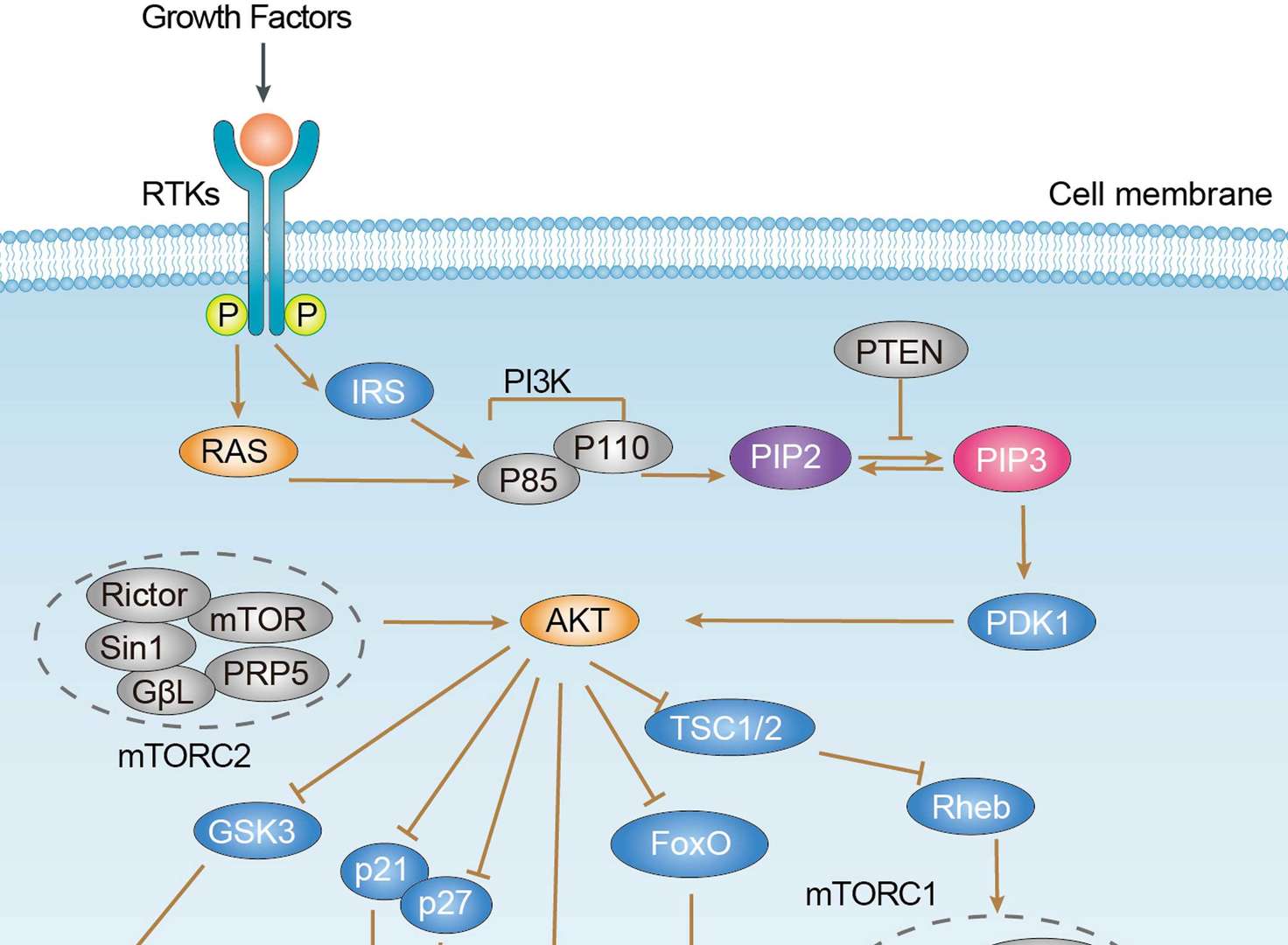 PI3K-Akt Signaling Pathway
PI3K-Akt Signaling Pathway