

Imalumab Overview
Introduction of Imalumab
Imalumab (as known as Bax69) is a novel recombinant, fully-human, monoclonal antibody (mAb) which was designed and developed to target the macrophage migration inhibitory factor, with potential immunomodulating, anti-inflammatory and antineoplastic activities. This drug was developed by Cytokine Pharma Sciences and Baxalta, which was purchased by Shire Pharmaceuticals. Phase I studies were completed in early 2016 and as of January 2017 it is being tested in Phase IIa clinical trials for metastatic colorectal cancer. However, it is disappointing that there are no breakthrough and exciting clinical trial results for this monoclonal antibody. For example, a phase I/II trial in patients with malignant ascites was terminated in 2016 and Baxalta terminated a phase II trial in Colorectal cancer (Combination therapy, Second-line therapy or greater, Metastatic disease) in USA, Spain on 02 Jun 2017.
Mechanism of Action of Imalumab
MIF was originally discovered in 1966 as a lymphokine, derived from activated T-cells, that inhibited the random migration of macrophages in vitro and was shown to be involved in the mechanism of delayed-type hypersensitivity. Currently, it is known that MIF is a widely expressed and pleiotropic cytokine that functions as a critical upstream mediator of innate immunity and promotes numerous pathophysiological processes, such as glomerulonephritis, arthritis, experimental autoimmune encephalomyelitis (EAE), experimental autoimmune myocarditis (EAM), gram-negative and gram-positive sepsis, colitis, asthma diabetes, and pancreatitis. MIF is produced and secreted primarily by immune cells, such as lymphocytes, Mø, DC, neutrophils and pituitary cells. MIF secretion is tightly regulated by stress and immune stimuli, including endotoxins, inflammatory cytokines (interferon (IFN)-γ, tumor necrosis factor (TNF)-α) and glucocorticoids. Once secreted, MIF exhibits a broad range of immune and inflammatory activities, including the induction of inflammatory cytokines such as TNF-α, IFN-γ, interleukin (IL)-1β, IL-12, IL-6 and, CXCL8 (also known as IL-8), among others. In addition, MIF counter-regulates the immunosuppressive effects of glucocorticoids, and it sustains macrophage proinflammatory functions by inhibiting p53. Studies showing that MIF-specific blocking antibodies could attenuate endotoxic shock confirmed the critical role that MIF plays in innate immunity. MIF's unique biological activities have the potential to contribute to an in vivo microenvironment favoring tumor growth and invasiveness. These functional activities include: tumor suppressor downregulation, COX-2 and PGE2 upregulation, potent induction of angiogenesis and enhanced tumor growth, proliferation and invasiveness. At the same time, MIF directly promotes tumorigenesis by inhibiting p53 accumulation. Imalumab is a novel recombinant, fully human, monoclonal antibody targeting MIF with potential immunomodulating, anti-inflammatory and antineoplastic activities. Upon intravenous administration, imalumab binds to MIF, blocking its activity and preventing the MIF-mediated secretion of certain cytokines, including interleukin-1 beta and tumor necrosis factor-alpha. This may lead to an inhibition of cancer cell proliferation in MIF-overexpressing tumor cells. Preclinical data demonstrated imalumab has antitumor activity and acceptable toxicity.
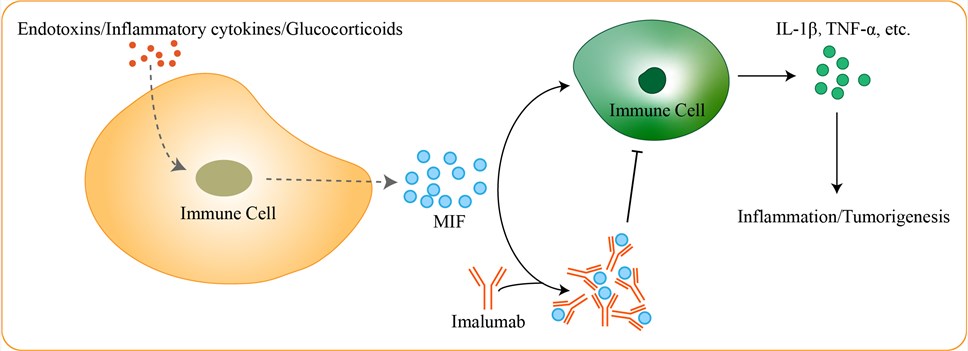 Fig 1. Mechanism of Action of Imalumab
Fig 1. Mechanism of Action of Imalumab
What We Provide
Therapeutic Antibody
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

