

Lexatumumab Overview
Introduction of Lexatumumab
Lexatumumab, previously called HGS-ETR2, is a complete monoclonal antibody targeting tumor necrosis factor receptor superfamily member 10B (TNFRSF10B). It was developed by Human Genome Science (HGS) through a collaboration with Cambridge Antibody Technology and has been investigated in the treatment of various cancers. Lexatumumab’s targeted molecule TNFRSF10B, also known as tumor necrosis factor-alpha (TNF-alpha)-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) and death receptor 5 (DR5), is a member of the TNF receptor superfamily. TNFRSF10B is a cell surface membrane receptor for TNFSF10/TRAIL toxic ligand and induces cell apoptosis when it binds to TRAIL. Lexatumumab has been investigated in the clinical trials of pediatric solid tumors, triple-negative breast cancer, malignant pleural mesothelioma (MPM) and pancreatic cancer. Studies have found that there was a marked synergistic apoptosis induced by lexatumumab in combination with epirubicin or pirarubicin in human renal cell carcinoma cells. In addition, lexatumumab has been reported to synergize with the inhibitor of apoptosis (IAP) to reduce cell viability and to induce apoptosis in some rhabdomyosarcoma (RMS) cell lines in a highly synergistic manner (combination index <0.1). Lexatumumab also has been observed to induce an additive or synergistic effect on cell death in the various melanoma cell lines in combination with dacarbazine (DTIC). PhaseⅠtrial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors have found this drug seems to mediate some clinical activity in pediatric solid tumors and may work with radiation to enhance antitumor effects. Recently study about pancreatic carcinoma has demonstrated lexatumumab combining with focal adhesion kinase inhibitor PF573228 synergistically induce cancer cells apoptosis.
Mechanism of Action of Lexatumumab
TNFRSF10B is one of the five death receptors, which are stimulated by the external apoptotic initiator and transmit apoptotic signals through different signaling systems to induce apoptosis. Just like other members of TFN superfamily, the extracellular part of TNFRSF10B is a cysteine-rich region, and the intracellular region is a death domain with hydrolytic function, which is responsible for the trigger of intracellular apoptosis signal. When TNFRSF10B binds to its ligand, Fas-associated death domain (FADD) is recruited. FADD forms the death induced signal complex (DISC) with procaspase 8 through its N-terminal death effector domain (DED), stimulating and activating caspase-8 and caspase-10. After activation, caspase-8 catalyzes caspase protease to produce cascade amplification reaction and finally plays the biological function by activating its effector caspase-3. Diseases associated with TNFRSF10B include squamous cell carcinoma, head and neck, and diffuse infiltrative lymphocytosis syndrome. And the related pathways TNFRSF10B involved in include gene expression and protein kinase B (Akt) signaling. TNFRSF10B is more widely expressed on the surface of tumor cells than normal cells, and when it binds to related ligands, it can selectively kill a variety of tumor cells and is less toxic to normal cells. Therefore, TNFRSF10B has become the most ideal target molecule for developing anti-tumor specific monoclonal antibody drugs. Lexatumumab, an Immunoglobulin G4κ (IgG4κ) type antibody, is one of the experimental agonist targeting TNFRSF10B. This drug is designed to mimic the natural ligand TRAIL, binding to TNFRSF10B and triggering the apoptosis of tumor cells.
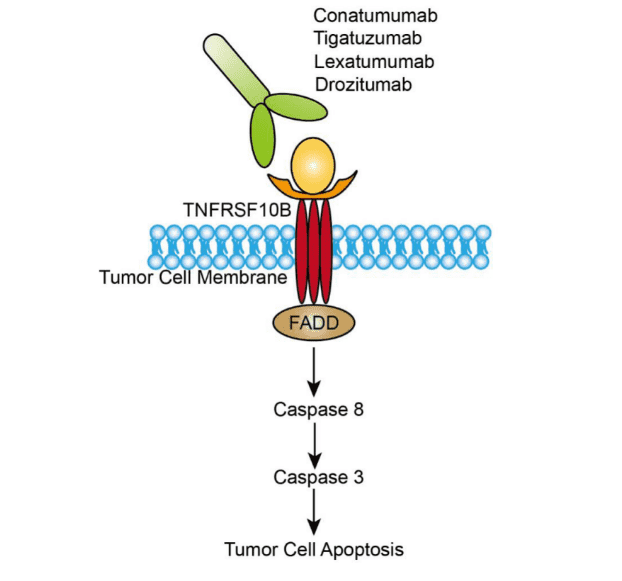 Fig.1 Mechanism of action of Lexatumumab
Fig.1 Mechanism of action of Lexatumumab
Table 1. Clinical Projects of Lexatumumab *
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03008278 | Recruiting | Gastroesophageal Junction Adenocarcinoma | National Cancer Institute (NCI) | November 1, 2018 |
| NCT03694002 | Recruiting | Locally Advanced Thymic Carcinoma, Metastatic Thymic Carcinoma, Recurrent Thymic Carcinoma, Unresectable Thymic Carcinoma | Southwest Oncology Group | October 3, 2018 |
What We Provide
Therapeutic Antibody
Lexatumumab
We provide high-quality Lexatumumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, notfor diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?term=Lexatumumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

